Rituximab therapy for chronic and refractory immune thrombocytopenic purpura: a long-term follow-up analysis
- PMID: 17874322
- PMCID: PMC2040174
- DOI: 10.1007/s00277-007-0317-3
Rituximab therapy for chronic and refractory immune thrombocytopenic purpura: a long-term follow-up analysis
Abstract
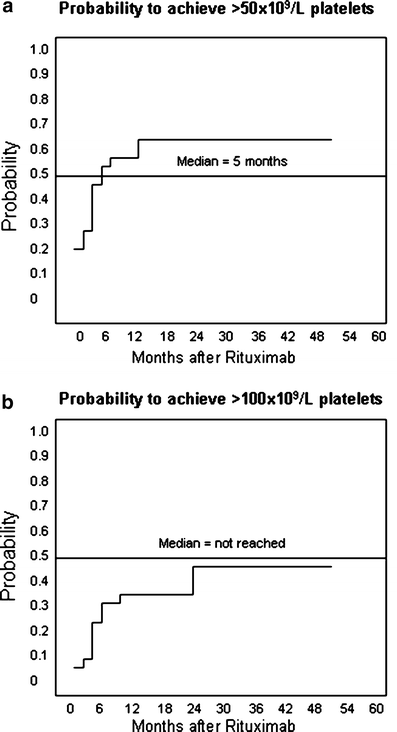
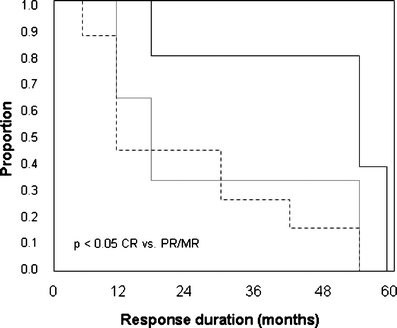
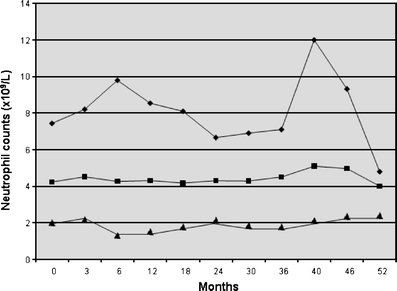
The aim of this study was to evaluate the long-term response to rituximab in patients with chronic and refractory immune thrombocytopenic purpura (ITP). Adults with ITP fail to respond to conventional therapies in almost 30% of cases, developing a refractory disease. Rituximab has been successfully used in these patients. We used rituximab at 375 mg/m2, IV, weekly for a total of four doses in 18 adult patients. Complete remission (CR) was considered if the platelet count was >100 x 10(9)/l, partial remission (PR) if platelets were >50 x 10(9)/l, minimal response (MR) if the platelet count was >30 x 10(9)/l and <50 x 10(9)/l, and no response if platelet count remained unchanged. Response was classified as sustained (SR) when it was stable for a minimum of 6 months. Median age was 43.5 years (range, 17 to 70). Median platelet count at baseline was 12.5 x 10(9)/l (range, 3.0 to 26.3). CR was achieved in five patients (28%), PR in five (28%), MR in four (22%), and two patients were classified as therapeutic failures (11%). Two additional patients were lost to follow-up. The median time between rituximab therapy and response was 14 weeks (range, 4 to 32). SR was achieved in 12 patients (67%). There were no severe adverse events during rituximab therapy. During follow-up (median, 26 months; range, 12 to 59), no other immunosuppressive drugs were used. In conclusion, rituximab therapy is effective and safe in adult patients with chronic and refractory ITP. Overall response rate achieved is high, long term, and with no risk of adverse events.
Figures
Similar articles
-
Rituximab chimeric anti-CD20 monoclonal antibody treatment for adult refractory idiopathic thrombocytopenic purpura.Am J Hematol. 2005 Apr;78(4):275-80. doi: 10.1002/ajh.20276. Am J Hematol. 2005. PMID: 15795920
-
Low rate of long-lasting remissions after successful treatment of immune thrombocytopenic purpura with rituximab.Ann Hematol. 2007 Oct;86(10):711-7. doi: 10.1007/s00277-007-0335-1. Epub 2007 Jul 11. Ann Hematol. 2007. PMID: 17622529
-
Rituximab therapy in adult patients with relapsed or refractory immune thrombocytopenic purpura: long-term follow-up results.Eur J Haematol. 2008 Sep;81(3):165-9. doi: 10.1111/j.1600-0609.2008.01100.x. Epub 2008 May 27. Eur J Haematol. 2008. PMID: 18510702
-
Anti B-cell therapy against refractory thrombocytopenia in SLE and MCTD patients: long-term follow-up and review of the literature.Lupus. 2013 Jun;22(7):664-74. doi: 10.1177/0961203313485489. Epub 2013 Apr 23. Lupus. 2013. PMID: 23612795 Review.
-
Rituximab treatment for chronic refractory idiopathic thrombocytopenic purpura.Crit Rev Oncol Hematol. 2008 Jan;65(1):21-31. doi: 10.1016/j.critrevonc.2007.06.007. Epub 2007 Jul 30. Crit Rev Oncol Hematol. 2008. PMID: 17681784 Review.
Cited by
-
Comparison of Response to Rituximab Therapy in Adults with Refractory Symptomatic Immune Thrombocytopenia According to the Presence of Accessory Spleen.Hematol Rep. 2022 Jul 5;14(3):222-227. doi: 10.3390/hematolrep14030030. Hematol Rep. 2022. PMID: 35893154 Free PMC article.
-
Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia.Blood. 2012 Jun 21;119(25):5989-95. doi: 10.1182/blood-2011-11-393975. Epub 2012 May 7. Blood. 2012. PMID: 22566601 Free PMC article.
-
Favorable response of chronic refractory immune thrombocytopenic purpura to mesenchymal stem cells.Stem Cells Dev. 2012 Feb 10;21(3):497-502. doi: 10.1089/scd.2011.0231. Epub 2011 Aug 12. Stem Cells Dev. 2012. PMID: 21711157 Free PMC article.
-
Repeated courses of rituximab in chronic ITP: Three different regimens.Am J Hematol. 2009 Oct;84(10):661-5. doi: 10.1002/ajh.21512. Am J Hematol. 2009. PMID: 19731307 Free PMC article. Clinical Trial.
-
Clinical Practice Updates in the Management Of Immune Thrombocytopenia.P T. 2017 Dec;42(12):756-763. P T. 2017. PMID: 29234214 Free PMC article.
References
-
- None
- Ahn ER, Bidot CJ, Fontana V, Dudkiewicz P, Jy W, Hortsman L et al (2005) Rituximab therapy induces long lasting clinical remissions and reduction of anti-platelet glycoprotein autoantibodies in patients with chronic immune thrombocytopenic purpura (ITP). Blood 6:725
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '17200219', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/17200219/'}]}
- Arnold DM, Dentali F, Crowther MA, Meyer RM, Cook RJ, Sigouin C, Fraser GA, Lim W, Kelton JG (2007) Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med 146:25–33 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1182/blood-2005-08-3518', 'is_inner': False, 'url': 'https://doi.org/10.1182/blood-2005-08-3518'}, {'type': 'PMC', 'value': 'PMC1895391', 'is_inner': False, 'url': 'https://pmc.ncbi.nlm.nih.gov/articles/PMC1895391/'}, {'type': 'PubMed', 'value': '16352811', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16352811/'}]}
- Bennett CM, Rogers ZR, Kinnamon DD, Bussel JB, Mahoney DH, Abshire TC, Sawaf H, Moore TB, Loh ML, Glader BE, McCarthy MC, Mueller BU, Olson TA, Lorenzana AN, Mentzer WC, Buchanan GR, Feldman HA, Neufeld EJ (2006) Prospective phase 1/2 study of rituximab in childhood and adolescent chronic immune thrombocytopenic purpura. Blood 107:2639–2642 - PMC - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1002/ajh.20276', 'is_inner': False, 'url': 'https://doi.org/10.1002/ajh.20276'}, {'type': 'PubMed', 'value': '15795920', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15795920/'}]}
- Braendstrup P, Bjerrum OW, Nielsen OJ, Jensen BA, Clausen NT, Hansen PB, Andersen I, Schmidt K, Andersen TM, Peterslund NA, Birgens HS, Plesner T, Pedersen BB, Hasselbalch HC (2005) Rituximab chimeric anti-CD20 monoclonal antibody treatment for adult refractory idiopathic thrombocytopenic purpura. Am J Hematol 78:275–280 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1056/NEJMra010501', 'is_inner': False, 'url': 'https://doi.org/10.1056/nejmra010501'}, {'type': 'PubMed', 'value': '11919310', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11919310/'}]}
- Cines DB, Blanchette VS (2002) Immune thrombocytopenic purpura. N Engl J Med 346:995–1008 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

