Antimalarial drug quality in Africa
- PMID: 17875107
- PMCID: PMC2653781
- DOI: 10.1111/j.1365-2710.2007.00847.x
Antimalarial drug quality in Africa
Abstract
Background and objective: There are several reports of sub-standard and counterfeit antimalarial drugs circulating in the markets of developing countries; we aimed to review the literature for the African continent.
Methods: A search was conducted in PubMed in English using the medical subject headings (MeSH) terms: 'Antimalarials/analysis'[MeSH] OR 'Antimalarials/standards'[MeSH] AND 'Africa'[MeSH]' to include articles published up to and including 26 February 2007. Data were augmented with reports on the quality of antimalarial drugs in Africa obtained from colleagues in the World Health Organization. We summarized the data under the following themes: content and dissolution; relative bioavailability of antimalarial products; antimalarial stability and shelf life; general tests on pharmaceutical dosage forms; and the presence of degradation or unidentifiable impurities in formulations.
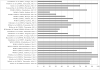
Results and discussion: The search yielded 21 relevant peer-reviewed articles and three reports on the quality of antimalarial drugs in Africa. The literature was varied in the quality and breadth of data presented, with most bioavailability studies poorly designed and executed. The review highlights the common finding in drug quality studies that (i) most antimalarial products pass the basic tests for pharmaceutical dosage forms, such as the uniformity of weight for tablets, (ii) most antimalarial drugs pass the content test and (iii) in vitro product dissolution is the main problem area where most drugs fail to meet required pharmacopoeial specifications, especially with regard to sulfadoxine-pyrimethamine products. In addition, there are worryingly high quality failure rates for artemisinin monotherapies such as dihydroartemisinin (DHA); for instance all five DHA sampled products in one study in Nairobi, Kenya, were reported to have failed the requisite tests.
Conclusions: There is an urgent need to strengthen pharmaceutical management systems such as post-marketing surveillance and the broader health systems in Africa to ensure populations in the continent have access to antimalarial drugs that are safe, of the highest quality standards and that retain their integrity throughout the distribution chain through adequate enforcement of existing legislation and enactment of new ones if necessary, and provision of the necessary resources for drug quality assurance.
Figures

References
-
- WHO . Effective drug regulation: what can countries do? Geneva: 1999. pp. 1–53.
-
- MCA . Towards safe medicines. A guide to the control of safety, quality and efficacy of human medicines in the United Kingdom. Revised edition 1997 Medicines Control Agency (MCA); London: 1997. pp. 1–93.
-
- Shah RR. Thalidomide, drug safety and early drug regulation in the UK. Adverse Drug Reaction and Toxicology Review. 2001;20:199–255. - PubMed
-
- Murray CJL, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269–1276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

