GATA elements control repression of cardiac troponin I promoter activity in skeletal muscle cells
- PMID: 17875210
- PMCID: PMC2045674
- DOI: 10.1186/1471-2199-8-78
GATA elements control repression of cardiac troponin I promoter activity in skeletal muscle cells
Abstract
Background: We reported previously that the cardiac troponin I (cTnI) promoter drives cardiac-specific expression of reporter genes in cardiac muscle cells and in transgenic mice, and that disruption of GATA elements inactivates the cTnI promoter in cultured cardiomyocytes. We have now examined the role of cTnI promoter GATA elements in skeletal muscle cells.
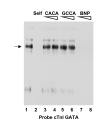
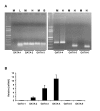
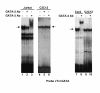
Results: Mutation or deletion of GATA elements induces a strong transcriptional activation of the cTnI promoter in regenerating skeletal muscle and in cultured skeletal muscle cells. Electrophoretic mobility shift assays show that proteins present in nuclear extracts of C2C12 muscle cells bind the GATA motifs present in the cTnI promoter. However, GATA protein complex formation is neither reduced nor supershifted by antibodies specific for GATA-2, -3 and -4, the only GATA transcripts present in muscle cells.
Conclusion: These findings indicate that the cTnI gene promoter is repressed in skeletal muscle cells by GATA-like factors and open the way to further studies aimed at identifying these factors.
Figures

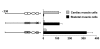

References
-
- Dhar M, Mascareno EM, Siddiqui MA. Two distinct factor-binding DNA elements in cardiac myosin light chain 2 gene are essential for repression of its expression in skeletal muscle. Isolation of a cDNA clone for repressor protein Nished. J Biol Chem. 1997;272:18490–18497. doi: 10.1074/jbc.272.29.18490. - DOI - PubMed
-
- Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76:371–423. - PubMed
-
- Vitadello M, Schiaffino MV, Picard A, Scarpa M, Schiaffino S. Gene transfer in regenerating muscle. Hum Gene Ther. 1994;5:11–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

