N-cofilin is associated with neuronal migration disorders and cell cycle control in the cerebral cortex
- PMID: 17875668
- PMCID: PMC1973148
- DOI: 10.1101/gad.434307
N-cofilin is associated with neuronal migration disorders and cell cycle control in the cerebral cortex
Abstract
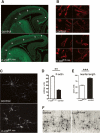
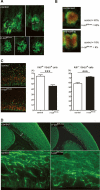
Many neuronal disorders such as lissencephaly, epilepsy, and schizophrenia are caused by the abnormal migration of neurons in the developing brain. The role of the actin cytoskeleton in neuronal migration disorders has in large part remained elusive. Here we show that the F-actin depolymerizing factor n-cofilin controls cell migration and cell cycle progression in the cerebral cortex. Loss of n-cofilin impairs radial migration, resulting in the lack of intermediate cortical layers. Neuronal progenitors in the ventricular zone show increased cell cycle exit and exaggerated neuronal differentiation, leading to the depletion of the neuronal progenitor pool. These results demonstrate that mutations affecting regulators of the actin cytoskeleton contribute to the pathology of cortex development.
Figures



References
-
- Adams M.E., Minamide L.S., Duester G., Bamburg J.R., Minamide L.S., Duester G., Bamburg J.R., Duester G., Bamburg J.R., Bamburg J.R. Nucleotide sequence and expression of a cDNA encoding chick brain actin depolymerizing factor. Biochemistry. 1990;29:7414–7420. - PubMed
-
- Anderson S.A., Eisenstat D.D., Shi L., Rubenstein J.L., Eisenstat D.D., Shi L., Rubenstein J.L., Shi L., Rubenstein J.L., Rubenstein J.L. Interneuron migration from basal forebrain to neocortex: Dependence on Dlx genes. Science. 1997;278:474–476. - PubMed
-
- Ayala R., Shu T., Tsai L.H., Shu T., Tsai L.H., Tsai L.H. Trekking across the brain: The journey of neuronal migration. Cell. 2007;128:29–43. - PubMed
-
- Cappello S., Attardo A., Wu X., Iwasato T., Itohara S., Wilsch-Brauninger M., Eilken H.M., Rieger M.A., Schroeder T.T., Huttner W.B., Attardo A., Wu X., Iwasato T., Itohara S., Wilsch-Brauninger M., Eilken H.M., Rieger M.A., Schroeder T.T., Huttner W.B., Wu X., Iwasato T., Itohara S., Wilsch-Brauninger M., Eilken H.M., Rieger M.A., Schroeder T.T., Huttner W.B., Iwasato T., Itohara S., Wilsch-Brauninger M., Eilken H.M., Rieger M.A., Schroeder T.T., Huttner W.B., Itohara S., Wilsch-Brauninger M., Eilken H.M., Rieger M.A., Schroeder T.T., Huttner W.B., Wilsch-Brauninger M., Eilken H.M., Rieger M.A., Schroeder T.T., Huttner W.B., Eilken H.M., Rieger M.A., Schroeder T.T., Huttner W.B., Rieger M.A., Schroeder T.T., Huttner W.B., Schroeder T.T., Huttner W.B., Huttner W.B., et al. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat. Neurosci. 2006;9:1099–1107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
