The prepattern transcription factor Irx3 directs nephron segment identity
- PMID: 17875669
- PMCID: PMC1973149
- DOI: 10.1101/gad.450707
The prepattern transcription factor Irx3 directs nephron segment identity
Abstract
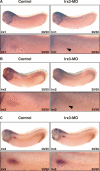
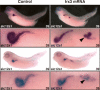
The nephron, the basic structural and functional unit of the vertebrate kidney, is organized into discrete segments, which are composed of distinct renal epithelial cell types. Each cell type carries out highly specific physiological functions to regulate fluid balance, osmolarity, and metabolic waste excretion. To date, the genetic basis of regionalization of the nephron has remained largely unknown. Here we show that Irx3, a member of the Iroquois (Irx) gene family, acts as a master regulator of intermediate tubule fate. Comparative studies in Xenopus and mouse have identified Irx1, Irx2, and Irx3 as an evolutionary conserved subset of Irx genes, whose expression represents the earliest manifestation of intermediate compartment patterning in the developing vertebrate nephron discovered to date. Intermediate tubule progenitors will give rise to epithelia of Henle's loop in mammals. Loss-of-function studies indicate that irx1 and irx2 are dispensable, whereas irx3 is necessary for intermediate tubule formation in Xenopus. Furthermore, we demonstrate that misexpression of irx3 is sufficient to direct ectopic development of intermediate tubules in the Xenopus mesoderm. Taken together, irx3 is the first gene known to be necessary and sufficient to specify nephron segment fate in vivo.
Figures


References
-
- Aldaz S., Morata G., Azpiazu N., Morata G., Azpiazu N., Azpiazu N. The Pax-homeobox gene eyegone is involved in the subdivision of the thorax of Drosophila. Development. 2003;130:4473–4482. - PubMed
-
- Allanson J.E., Hunter A.G., Mettler G.S., Jimenez C., Hunter A.G., Mettler G.S., Jimenez C., Mettler G.S., Jimenez C., Jimenez C. Renal tubular dysgenesis: A not uncommon autosomal recessive syndrome: A review. Am. J. Med. Genet. 1992;43:811–814. - PubMed
-
- Bao Z.Z., Bruneau B.G., Seidman J.G., Seidman C.E., Cepko C.L., Bruneau B.G., Seidman J.G., Seidman C.E., Cepko C.L., Seidman J.G., Seidman C.E., Cepko C.L., Seidman C.E., Cepko C.L., Cepko C.L. Regulation of chamber-specific gene expression in the developing heart by Irx4. Science. 1999;283:1161–1164. - PubMed
-
- Bellefroid E.J., Kobbe A., Gruss P., Pieler T., Gurdon J.B., Papalopulu N., Kobbe A., Gruss P., Pieler T., Gurdon J.B., Papalopulu N., Gruss P., Pieler T., Gurdon J.B., Papalopulu N., Pieler T., Gurdon J.B., Papalopulu N., Gurdon J.B., Papalopulu N., Papalopulu N. Xiro3 encodes a Xenopus homolog of the Drosophila Iroquois genes and functions in neural specification. EMBO J. 1998;17:191–203. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
