The transcription factor XBP-1 is essential for the development and survival of dendritic cells
- PMID: 17875675
- PMCID: PMC2118458
- DOI: 10.1084/jem.20070525
The transcription factor XBP-1 is essential for the development and survival of dendritic cells
Abstract
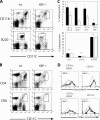
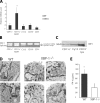
Dendritic cells (DCs) play a critical role in the initiation, maintenance, and resolution of an immune response. DC survival is tightly controlled by extracellular stimuli such as cytokines and Toll-like receptor (TLR) signaling, but the intracellular events that translate such extracellular stimuli into life or death for the DC remain poorly understood. The endoplasmic reticulum (ER) stress, or unfolded protein response (UPR), is a signaling pathway that is activated when unfolded proteins accumulate in the ER. The most conserved arm of the UPR involves IRE1alpha, an ER transmembrane kinase and endoribonuclease that activates the transcription factor XBP-1 to maintain ER homeostasis and prevent activation of cell death pathways caused by sustained ER stress. We report that XBP-1 is essential for DC development and survival. Lymphoid chimeras lacking XBP-1 possessed decreased numbers of both conventional and plasmacytoid DCs with reduced survival both at baseline and in response to TLR signaling. Overexpression of XBP-1 in hematopoietic progenitors rescued and enhanced DC development. Remarkably, in contrast to other cell types we have examined, the XBP-1 pathway was constitutively activated in immature DCs.
Figures


References
-
- Reimold, A.M., N.N. Iwakoshi, J. Manis, P. Vallabhajosyula, E. Szomolanyi-Tsuda, E.M. Gravallese, D. Friend, M.J. Grusby, F. Alt, and L.H. Glimcher. 2001. Plasma cell differentiation requires transcription factor XBP-1. Nature. 412:300–307. - PubMed
-
- Lipson, K.L., S.G. Fonseca, S. Ishigaki, L.X. Nguyen, E. Foss, R. Bortell, A.A. Rossini, and F. Urano. 2006. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 4:245–254. - PubMed
-
- Koumenis, C. 2006. ER stress, hypoxia tolerance and tumor progression. Curr. Mol. Med. 6:55–69. - PubMed
-
- Paschen, W., and T. Mengesdorf. 2005. Endoplasmic reticulum stress response and neurodegeneration. Cell Calcium. 38:409–415. - PubMed
-
- Liou, H.C., M.R. Boothby, P.W. Finn, R. Davidon, N. Nabavi, N.J. Zeleznik-Le, J.P. Ting, and L.H. Glimcher. 1990. A new member of the leucine zipper class of proteins that binds to the HLA DR alpha promoter. Science. 247:1581–1584. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

