Myosin IIA is required for cytolytic granule exocytosis in human NK cells
- PMID: 17875677
- PMCID: PMC2118468
- DOI: 10.1084/jem.20071143
Myosin IIA is required for cytolytic granule exocytosis in human NK cells
Abstract
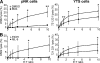
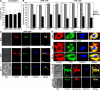
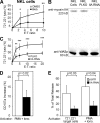
Natural killer (NK) cell cytotoxicity involves the formation of an activating immunological synapse (IS) between the effector and target cell through which granzymes and perforin contained in lytic granules are delivered to the target cell via exocytosis. Inhibition of nonmuscle myosin II in human NK cells with blebbistatin or ML-9 impaired neither effector-target cell conjugation nor formation of a mature activating NK cell IS (NKIS; formation of an actin ring and polarization of the microtubule-organizing center and cytolytic granules to the center of the ring). However, membrane fusion of lytic granules, granzyme secretion, and NK cell cytotoxicity were all effectively blocked. Specific knockdown of the myosin IIA heavy chain by RNA interference impaired cytotoxicity, membrane fusion of lytic granules, and granzyme secretion. Thus, myosin IIA is required for a critical step between NKIS formation and granule exocytosis.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

