Targeting human medulloblastoma: oncolytic virotherapy with myxoma virus is enhanced by rapamycin
- PMID: 17875723
- PMCID: PMC4380180
- DOI: 10.1158/0008-5472.CAN-07-1214
Targeting human medulloblastoma: oncolytic virotherapy with myxoma virus is enhanced by rapamycin
Abstract
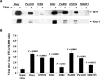
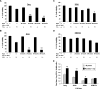
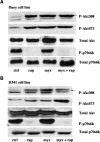
We have shown previously the oncolytic potential of myxoma virus in a murine xenograft model of human glioma. Here, we show that myxoma virus used alone or in combination with rapamycin is effective and safe when used in experimental models of medulloblastoma in vitro and in vivo. Nine of 10 medulloblastoma cell lines tested were susceptible to lethal myxoma virus infection, and pretreatment of cells with rapamycin increased the extent of in vitro oncolysis. Intratumoral injection of live myxoma virus when compared with control inactivated virus prolonged survival in D341 and Daoy orthotopic human medulloblastoma xenograft mouse models [D341 median survival: 21 versus 12.5 days; P = 0.0008; Daoy median survival: not reached (three of five mice apparently "cured" after 223 days) versus 75 days; P = 0.0021]. Rapamycin increased the extent of viral oncolysis, "curing" most Daoy tumor-bearing mice and reducing or eliminating spinal cord and ventricle metastases. Rapamycin enhanced tumor-specific myxoma virus replication in vivo and prolonged survival of D341 tumor-bearing mice (median survival of mice treated with live virus (LV) and rapamycin, versus LV alone, versus rapamycin alone, versus inactivated virus: 25 days versus 19, 13, and 11 days, respectively; P < 0.0001). Rapamycin increased the levels of constitutively activated Akt in Daoy and D341 cells, which may explain its ability to enhance myxoma virus oncolysis. These observations suggest that myxoma virus may be an effective oncolytic agent against medulloblastoma and that combination therapy with signaling inhibitors that modulate activity of the phosphatidylinositol 3-kinase/Akt pathway will further enhance the oncolytic potential of myxoma virus.
Figures



References
-
- Walker DA, Wilne S. Treatment of medulloblastoma in young children. Lancet Oncol. 2005;6:541–2. - PubMed
-
- Ribi K, Relly C, Landolt MA, et al. Outcome of medulloblastoma in children: long-term complications and quality of life. Neuropediatrics. 2005;36:357–65. - PubMed
-
- Gilbertson RJ. Medulloblastoma: signalling a change in treatment. Lancet Oncol. 2004;5:209–18. - PubMed
-
- Louis DN, Pomeroy SL, Cairncross JG. Focus on central nervous system neoplasia. Cancer Cell. 2002;1:125–8. - PubMed
-
- Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

