The EphA4 receptor regulates dendritic spine remodeling by affecting beta1-integrin signaling pathways
- PMID: 17875741
- PMCID: PMC2064660
- DOI: 10.1083/jcb.200610139
The EphA4 receptor regulates dendritic spine remodeling by affecting beta1-integrin signaling pathways
Abstract
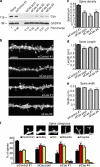
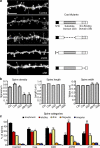
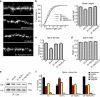
Remodeling of dendritic spines is believed to modulate the function of excitatory synapses. We previously reported that the EphA4 receptor tyrosine kinase regulates spine morphology in hippocampal pyramidal neurons, but the signaling pathways involved were not characterized (Murai, K.K., L.N. Nguyen, F. Irie, Y. Yamaguchi, and E.B. Pasquale. 2003. Nat. Neurosci. 6:153-160). In this study, we show that EphA4 activation by ephrin-A3 in hippocampal slices inhibits integrin downstream signaling pathways. EphA4 activation decreases tyrosine phosphorylation of the scaffolding protein Crk-associated substrate (Cas) and the tyrosine kinases focal adhesion kinase (FAK) and proline-rich tyrosine kinase 2 (Pyk2) and also reduces the association of Cas with the Src family kinase Fyn and the adaptor Crk. Consistent with this, EphA4 inhibits beta1-integrin activity in neuronal cells. Supporting a functional role for beta1 integrin and Cas inactivation downstream of EphA4, the inhibition of integrin or Cas function induces spine morphological changes similar to those associated with EphA4 activation. Furthermore, preventing beta1-integrin inactivation blocks the effects of EphA4 on spines. Our results support a model in which EphA4 interferes with integrin signaling pathways that stabilize dendritic spines, thus modulating synaptic interactions with the extracellular environment.
Figures


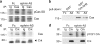



References
-
- Arnaout, M.A., B. Mahalingam, and J.P. Xiong. 2005. Integrin structure, allostery, and bidirectional signaling. Annu. Rev. Cell Dev. Biol. 21:381–410. - PubMed
-
- Bazzoni, G., D.T. Shih, C.A. Buck, and M.E. Hemler. 1995. Monoclonal antibody 9EG7 defines a novel beta 1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J. Biol. Chem. 270:25570–25577. - PubMed
-
- Bernard-Trifilo, J.A., E.A. Kramar, R. Torp, C.Y. Lin, E.A. Pineda, G. Lynch, and C.M. Gall. 2005. Integrin signaling cascades are operational in adult hippocampal synapses and modulate NMDA receptor physiology. J. Neurochem. 93:834–849. - PubMed
-
- Bonhoeffer, T., and R. Yuste. 2002. Spine motility. Phenomenology, mechanisms, and function. Neuron. 35:1019–1027. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

