Proteolysis and membrane capture of F-spondin generates combinatorial guidance cues from a single molecule
- PMID: 17875744
- PMCID: PMC2064656
- DOI: 10.1083/jcb.200702184
Proteolysis and membrane capture of F-spondin generates combinatorial guidance cues from a single molecule
Abstract
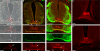
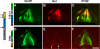
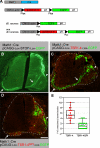
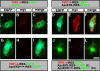
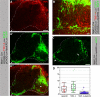
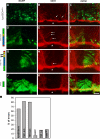
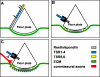
The formation of neuronal networks is governed by a limited number of guidance molecules, yet it is immensely complex. The complexity of guidance cues is augmented by posttranslational modification of guidance molecules and their receptors. We report here that cleavage of the floor plate guidance molecule F-spondin generates two functionally opposing fragments: a short-range repellent protein deposited in the membrane of floor plate cells and an adhesive protein that accumulates at the basement membrane. Their coordinated activity, acting respectively as a short-range repellant and a permissive short-range attractant, constricts commissural axons to the basement membrane beneath the floor plate cells. We further demonstrate that the repulsive activity of the inhibitory fragment of F-spondin requires its presentation by the lipoprotein receptor-related protein (LRP) receptors apolipoprotein E receptor 2, LRP2/megalin, and LRP4, which are expressed in the floor plate. Thus, proteolysis and membrane interaction coordinate combinatorial guidance signaling originating from a single guidance cue.
Figures



References
-
- Augsburger, A., A. Schuchardt, S. Hoskins, J. Dodd, and S. Butler. 1999. BMPs as mediators of roof plate repulsion of commissural neurons. Neuron. 24:127–141. - PubMed
-
- Bovolenta, P., and J. Dodd. 1990. Guidance of commissural growth cones at the floor plate in the embryonic rat spinal cord. Development. 109:435–447. - PubMed
-
- Brose, K., K.S. Bland, K.H. Wang, D. Arnott, W. Henzel, C.S. Goodman, M. Tessier-Lavigne, and T. Kidd. 1999. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 96:795–806. - PubMed
-
- Burstyn-Cohen, T., V. Tzarfaty, A. Frumkin, Y. Feinstein, E. Stoeckli, and A. Klar. 1999. F-spondin is required for accurate pathfinding of commissural axons at the floor plate. Neuron. 23:233–246. - PubMed
-
- Charron, F., E. Stein, J. Jeong, A.P. McMahon, and M. Tessier-Lavigne. 2003. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 113:11–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

