Reversion mutations in patients with leukocyte adhesion deficiency type-1 (LAD-1)
- PMID: 17875809
- PMCID: PMC2200806
- DOI: 10.1182/blood-2007-04-082552
Reversion mutations in patients with leukocyte adhesion deficiency type-1 (LAD-1)
Abstract
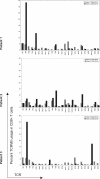
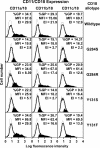
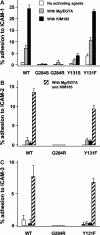
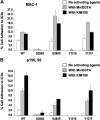
Leukocyte adhesion deficiency type-1 (LAD-1) is an autosomal recessive immunodeficiency caused by mutations in the beta2 integrin, CD18, that impair CD11/CD18 heterodimer surface expression and/or function. Absence of functional CD11/CD18 integrins on leukocytes, particularly neutrophils, leads to their incapacity to adhere to the endothelium and migrate to sites of infection. We studied 3 LAD-1 patients with markedly diminished neutrophil CD18 expression, each of whom had a small population of lymphocytes with normal CD18 expression (CD18(+)). These CD18(+) lymphocytes were predominantly cytotoxic T cells, with a memory/effector phenotype. Microsatellite analyses proved patient origin of these cells. Sequencing of T-cell subsets showed that in each patient one CD18 allele had undergone further mutation. Interestingly, all 3 patients were young adults with inflammatory bowel disease. Somatic reversions of inherited mutations in primary T-cell immunodeficiencies are typically associated with milder clinical phenotypes. We hypothesize that these somatic revertant CD18(+) cytotoxic T lymphocytes (CTLs) may have altered immune regulation. The discovery of 3 cases of reversion mutations in LAD-1 at one center suggests that this may be a relatively common event in this rare disease.
Figures


References
-
- Hirschhorn R, Yang DR, Puck JM, Huie ML, Jiang CK, Kurlandsky LE. Spontaneous in vivo reversion to normal of an inherited mutation in a patient with adenosine deaminase deficiency. Nat Genet. 1996;13:290–295. - PubMed
-
- Ariga T, Kondoh T, Yamaguchi K, et al. Spontaneous in vivo reversion of an inherited mutation in the Wiskott-Aldrich syndrome. J Immunol. 2001;166:5245–5249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

