Mutant p53 attenuates the SMAD-dependent transforming growth factor beta1 (TGF-beta1) signaling pathway by repressing the expression of TGF-beta receptor type II
- PMID: 17875924
- PMCID: PMC2169171
- DOI: 10.1128/MCB.00374-07
Mutant p53 attenuates the SMAD-dependent transforming growth factor beta1 (TGF-beta1) signaling pathway by repressing the expression of TGF-beta receptor type II
Abstract
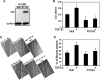
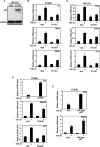
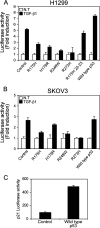
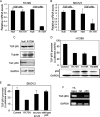
Both transforming growth factor beta (TGF-beta) and p53 have been shown to control normal cell growth. Acquired mutations either in the TGF-beta signaling pathway or in the p53 protein were shown to induce malignant transformation. Recently, cross talk between wild-type p53 and the TGF-beta pathway was observed. The notion that mutant p53 interferes with the wild-type p53-induced pathway and acts by a "gain-of-function" mechanism prompted us to investigate the effect of mutant p53 on the TGF-beta-induced pathway. In this study, we show that cells expressing mutant p53 lost their sensitivity to TGF-beta1, as observed by less cell migration and a reduction in wound healing. We found that mutant p53 attenuates TGF-beta1 signaling. This was exhibited by a reduction in SMAD2/3 phosphorylation and an inhibition of both the formation of SMAD2/SMAD4 complexes and the translocation of SMAD4 to the cell nucleus. Furthermore, we found that mutant p53 attenuates the TGF-beta1-induced transcription activity of SMAD2/3 proteins. In searching for the mechanism that underlies this attenuation, we found that mutant p53 reduces the expression of TGF-beta receptor type II. These data provide important insights into the molecular mechanisms that underlie mutant p53 "gain of function" pertaining to the TGF-beta signaling pathway.
Figures



References
-
- Aas, T., A. L. Borresen, S. Geisler, B. Smith-Sorensen, H. Johnsen, J. E. Varhaug, L. A. Akslen, and P. E. Lonning. 1996. Specific P53 mutations are associated with de novo resistance to doxorubicin in breast cancer patients. Nat. Med. 2:811-814. - PubMed
-
- Abdollah, S., M. Macias-Silva, T. Tsukazaki, H. Hayashi, L. Attisano, and J. L. Wrana. 1997. TbetaRI phosphorylation of Smad2 on Ser465 and Ser467 is required for Smad2-Smad4 complex formation and signaling. J. Biol. Chem. 272:27678-27685. - PubMed
-
- Blandino, G., A. J. Levine, and M. Oren. 1999. Mutant p53 gain of function: differential effects of different p53 mutants on resistance of cultured cells to chemotherapy. Oncogene 18:477-485. - PubMed
-
- Buganim, Y., E. Kalo, R. Brosh, H. Besserglick, I. Nachmany, Y. Rais, P. Stambolsky, X. Tang, M. Milyavsky, I. Shats, M. Kalis, N. Goldfinger, and V. Rotter. 2006. Mutant p53 protects cells from 12-O-tetradecanoylphorbol-13-acetate-induced death by attenuating activating transcription factor 3 induction. Cancer Res. 66:10750-10759. - PubMed
-
- Cadwell, C., and G. P. Zambetti. 2001. The effects of wild-type p53 tumor suppressor activity and mutant p53 gain-of-function on cell growth. Gene 277:15-30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
