The basic helix-loop-helix transcription factor NeuroD1 facilitates interaction of Sp1 with the secretin gene enhancer
- PMID: 17875929
- PMCID: PMC2169158
- DOI: 10.1128/MCB.00438-07
The basic helix-loop-helix transcription factor NeuroD1 facilitates interaction of Sp1 with the secretin gene enhancer
Abstract
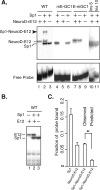
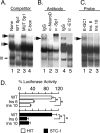
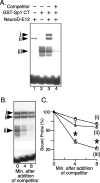
The basic helix-loop-helix transcription factor NeuroD1 is required for late events in neuronal differentiation, for maturation of pancreatic beta cells, and for terminal differentiation of enteroendocrine cells expressing the hormone secretin. NeuroD1-null mice demonstrated that this protein is essential for expression of the secretin gene in the murine intestine, and yet it is a relatively weak transcriptional activator by itself. The present study shows that Sp1 and NeuroD1 synergistically activate transcription of the secretin gene. NeuroD1, but not its widely expressed dimerization partner E12, physically interacts with the C-terminal 167 amino acids of Sp1, which include its DNA binding zinc fingers. NeuroD1 stabilizes Sp1 DNA binding to an adjacent Sp1 binding site on the promoter to generate a higher-order DNA-protein complex containing both proteins and facilitates Sp1 occupancy of the secretin promoter in vivo. NeuroD-dependent transcription of the genes encoding the hormones insulin and proopiomelanocortin is potentiated by lineage-specific homeodomain proteins. The stabilization of binding of the widely expressed transcription factor Sp1 to the secretin promoter by NeuroD represents a distinct mechanism from other NeuroD target genes for increasing NeuroD-dependent transcription.
Figures




References
-
- Cong, Y.-S., J. Wen, and S. Bacchetti. 1999. The human telomerase catalytic subunit hTERT: organization of the gene and characterization of the promoter. Hum. Mol. Genet. 8:137-142. - PubMed
-
- Courey, A. J., and R. Tjian. 1988. Analysis of Sp1 in vivo reveals multiple transactivational domains, including a novel glutamine-rich activation motif. Cell 55:887-898. - PubMed
-
- Dumonteil, E., B. Laser, I. Constant, and J. Philippe. 1998. Differential regulation of the glucagon and insulin I gene promoters by basic helix-loop-helix transcription factors E47 and Beta2. J. Biol. Chem. 273:19945-19954. - PubMed
-
- Edlund, T., M. D. Walker, P. J. Barr, and W. J. Rutter. 1985. Cell-specific expression of the rat insulin gene: evidence for role of two distinct 5′ flanking elements. Science 230:912-916. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
