Time gap between oocyst shedding and antibody responses in mice infected with Cryptosporidium parvum
- PMID: 17876169
- PMCID: PMC2526318
- DOI: 10.3347/kjp.2007.45.3.225
Time gap between oocyst shedding and antibody responses in mice infected with Cryptosporidium parvum
Abstract
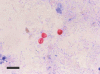
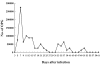
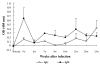
We observed the time gap between oocyst shedding and antibody responses in mice (3-week-old C57BL/6J females) infected with Cryptosporidium parvum. Oocyst shedding was verified by modified acid-fast staining. The individually collected mouse sera were assessed for C. parvum IgM and IgG antibodies by enzyme-linked immunosorbent assay from 5 to 25 weeks after infection. The results showed that C. parvum oocysts were shed from day 5 to 51 post-infection (PI). The IgM antibody titers to C. parvum peaked at week 5 PI, whereas the IgG antibody titers achieved maximum levels at week 25 PI. The results revealed that IgM responses to C. parvum infection occurred during the early stage of infection and overlapped with the oocyst shedding period, whereas IgG responses occurred during the late stage and was not correlated with oocyst shedding. Hence, IgM antibody detection may prove helpful for the diagnosis of acute cryptosporidiosis, and IgG antibody detection may prove effective for the detection of past infection and endemicity.
Figures
Similar articles
-
Concurrent response to challenge infection with Cryptosporidium parvum in immunosuppressed C57BL/6N mice.J Vet Sci. 2006 Mar;7(1):47-51. doi: 10.4142/jvs.2006.7.1.47. J Vet Sci. 2006. PMID: 16434849 Free PMC article.
-
Enzyme-linked immunosorbent assay for the detection of Cryptosporidium parvum IgG in the serum of cats.J Parasitol. 1997 Oct;83(5):957-60. J Parasitol. 1997. PMID: 9379309
-
Serum antibody response in lambs naturally and experimentally infected with Cryptosporidium parvum.Vet Parasitol. 1993 Oct;50(1-2):45-54. doi: 10.1016/0304-4017(93)90006-9. Vet Parasitol. 1993. PMID: 8291196
-
Innate and cell-mediated immune responses to Cryptosporidium parvum.Adv Parasitol. 1998;40:87-119. doi: 10.1016/s0065-308x(08)60118-9. Adv Parasitol. 1998. PMID: 9554071 Review.
-
Antibody-based immunotherapy of cryptosporidiosis.Adv Parasitol. 1998;40:121-49. doi: 10.1016/s0065-308x(08)60119-0. Adv Parasitol. 1998. PMID: 9554072 Review.
Cited by
-
Evaluation of a vaccine candidate isolated from Cryptosporidium parvum oocyst in mice.Vet World. 2022 Dec;15(12):2772-2784. doi: 10.14202/vetworld.2022.2772-2784. Epub 2022 Dec 5. Vet World. 2022. PMID: 36718331 Free PMC article.
-
Diagnostic biomarkers in murine Cryptosporidiosis: dose- and age-related infection.J Parasit Dis. 2017 Sep;41(3):831-836. doi: 10.1007/s12639-017-0898-2. Epub 2017 Feb 18. J Parasit Dis. 2017. PMID: 28848287 Free PMC article.
-
Anticryptosporidial action mechanisms of Launaea spinosa extracts in Cryptosporidium parvum experimentally infected mice in relation to its UHPLC-MS metabolite profile and biochemometric tools.PLoS One. 2025 Mar 3;20(3):e0317497. doi: 10.1371/journal.pone.0317497. eCollection 2025. PLoS One. 2025. PMID: 40029925 Free PMC article.
References
-
- Bonnin A, Dubremetz JF, Camerlynck P. Characterization and immunolocalization of an oocyst wall antigen of Cryptosporidium parvum (Protozoa: Apicomplexa) Parasitology. 1991;103:171–177. - PubMed
-
- Graczyk TK, Cranfield MR. Detection of Cryptosporidium-specific serum immunoglobulins in captive snakes by a polyclonal antibody in the indirect ELISA. Vet Res. 1997;28:131–142. - PubMed
-
- Lappin MR, Ungar B, Brown-Hahn B, Cooper CM, Spilker M, Thrall MA, Hill SL, Cheney J, Taton-Allen G. Enzyme-linked immunosorbent assay for the detection of Cryptosporidium parvum IgG in the serum of cats. J Parasitol. 1997;83:957–960. - PubMed
-
- Lazo A, Barriga OO, Redman DR, Bech-Nielsen S. Identification by transfer blot of antigens reactive in the enzyme-linked immunosorbent assay (ELISA) in rabbits immunized and a calf infected with Cryptosporidium sp. Vet Parasitol. 1986;21:151–163. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

