The complications of promiscuity: endocannabinoid action and metabolism
- PMID: 17876303
- PMCID: PMC2190010
- DOI: 10.1038/sj.bjp.0707456
The complications of promiscuity: endocannabinoid action and metabolism
Abstract
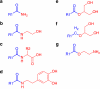
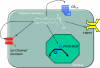
In this review, we present our understanding of the action and metabolism of endocannabinoids and related endogenous molecules. It is clear that the interactions between the multiple endocannabinoid-like molecules (ECLs) are highly complex, both at the level of signal transduction and metabolism. Thus, ECLs are a group of ligands active at 7-transmembrane and nuclear receptors, as well as transmitter-gated and ion channels. ECLs and their metabolites can converge on common endpoints (either metabolic or signalling) through contradictory or reinforcing pathways. We highlight the complexity of the endocannabinoid system, based on the promiscuous nature of ECLs and their metabolites, as well as the synthetic modulators of the endocannabinoid system.
Figures
Comment in
-
Cannabinoids and their actions.Br J Pharmacol. 2007 Nov;152(5):557-8. doi: 10.1038/sj.bjp.0707483. Epub 2007 Sep 24. Br J Pharmacol. 2007. PMID: 17891156 Free PMC article. No abstract available.
References
-
- Ahern GP. Activation of TRPV1 by the satiety factor oleoylethanolamide. J Biol Chem. 2003;278:30429–30434. - PubMed
-
- Akiyama TE, Sakai S, Lambert G, Nicol CJ, Matsusue K, Pimprale S, et al. Conditional disruption of the peroxisome proliferator-activated receptor γ gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux. Mol Cell Biol. 2002;22:2607–2619. - PMC - PubMed
-
- Alexander JP, Cravatt BF. The putative endocannabinoid transport blocker LY2183240 is a potent inhibitor of FAAH and several other brain serine hydrolases. J Am Chem Soc. 2006;128:9699–9704. - PubMed
-
- Alexander SPH, Hill SJ, Kendall DA. Synergistic interaction between glutamate analogues and histamine receptor-stimulated phosphoinositide turnover. Can J Physiol Pharmacol. 1994;72:533.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

