Targeted knockout and lacZ reporter expression of the mouse Tmhs deafness gene and characterization of the hscy-2J mutation
- PMID: 17876667
- PMCID: PMC2613174
- DOI: 10.1007/s00335-007-9049-x
Targeted knockout and lacZ reporter expression of the mouse Tmhs deafness gene and characterization of the hscy-2J mutation
Abstract
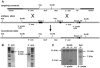
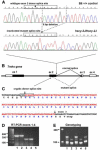
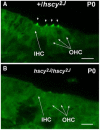
The Tmhs gene codes for a tetraspan transmembrane protein that is expressed in hair cell stereocilia. We previously showed that a spontaneous missense mutation of Tmhs underlies deafness and vestibular dysfunction in the hurry-scurry (hscy) mouse. Subsequently, mutations in the human TMHS gene were shown to be responsible for DFNB67, an autosomal recessive nonsyndromic deafness locus. Here we describe a genetically engineered null mutation of the mouse Tmhs gene (Tmhs ( tm1Kjn )) and show that its phenotype is identical to that of the hscy missense mutation, confirming the deleterious nature of the hscy cysteine-to-phenylalanine substitution. In the targeted null allele, the Tmhs promoter drives expression of a lacZ reporter gene. Visualization of beta-galactosidase activity in Tmhs ( tm1Kjn ) heterozygous mice indicates that Tmhs is highly expressed in the cochlear and vestibular hair cells of the inner ear. Expression is first detectable at E15.5, peaks around P0, decreases slightly at P6, and is absent by P15, a duration that supports the involvement of Tmhs in stereocilia development. Tmhs reporter gene expression also was detected in several cranial and cervical sensory ganglia, but not in the vestibular or spiral ganglia. We also describe a new nontargeted mutation of the Tmhs gene, hscy-2J, that causes abnormal splicing from a cryptic splice site within exon 2 and is predicted to produce a functionally null protein lacking 51 amino acids of the wild-type sequence.
Figures



Similar articles
-
A missense mutation in the previously undescribed gene Tmhs underlies deafness in hurry-scurry (hscy) mice.Proc Natl Acad Sci U S A. 2005 May 31;102(22):7894-9. doi: 10.1073/pnas.0500760102. Epub 2005 May 19. Proc Natl Acad Sci U S A. 2005. PMID: 15905332 Free PMC article.
-
Mutations of human TMHS cause recessively inherited non-syndromic hearing loss.J Med Genet. 2006 Aug;43(8):634-40. doi: 10.1136/jmg.2005.039834. Epub 2006 Feb 3. J Med Genet. 2006. PMID: 16459341 Free PMC article.
-
A missense mutation in the conserved C2B domain of otoferlin causes deafness in a new mouse model of DFNB9.Hear Res. 2007 Dec;234(1-2):21-8. doi: 10.1016/j.heares.2007.09.005. Epub 2007 Sep 29. Hear Res. 2007. PMID: 17967520 Free PMC article.
-
Mouse tales from Kresge: the deafness mouse.J Am Acad Audiol. 2003 Aug;14(6):296-301. J Am Acad Audiol. 2003. PMID: 14552423 Review.
-
Unravelling the genetics of deafness.Ann Otol Rhinol Laryngol Suppl. 1997 May;168:59-62. Ann Otol Rhinol Laryngol Suppl. 1997. PMID: 9153119 Review.
Cited by
-
Mechanisms in cochlear hair cell mechano-electrical transduction for acquisition of sound frequency and intensity.Cell Mol Life Sci. 2021 Jun;78(12):5083-5094. doi: 10.1007/s00018-021-03840-8. Epub 2021 Apr 19. Cell Mol Life Sci. 2021. PMID: 33871677 Free PMC article. Review.
-
Function and expression pattern of nonsyndromic deafness genes.Curr Mol Med. 2009 Jun;9(5):546-64. doi: 10.2174/156652409788488775. Curr Mol Med. 2009. PMID: 19601806 Free PMC article. Review.
-
Gene Therapy in Mouse Models of Deafness and Balance Dysfunction.Front Mol Neurosci. 2018 Aug 29;11:300. doi: 10.3389/fnmol.2018.00300. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30210291 Free PMC article.
-
Multiple plasma membrane reporters discern LHFPL5 region that blocks trafficking to the plasma membrane.Sci Rep. 2023 Feb 13;13(1):2528. doi: 10.1038/s41598-023-28045-w. Sci Rep. 2023. PMID: 36781873 Free PMC article.
-
Integrating the biophysical and molecular mechanisms of auditory hair cell mechanotransduction.Nat Commun. 2011 Nov 1;2:523. doi: 10.1038/ncomms1533. Nat Commun. 2011. PMID: 22045002 Free PMC article. Review.
References
-
- Adato A, Michel V, Kikkawa Y, Reiners J, Alagramam KN, et al. Interactions in the network of Usher syndrome type 1 proteins. Hum Mol Genet. 2005;14:347–356. - PubMed
-
- Anderson DJ, Groves A, Lo L, Ma Q, Rao M, et al. Cell lineage determination and the control of neuronal identity in the neural crest. Cold Spring Harb Symp Quant Biol. 1997;62:493–504. - PubMed
-
- Anniko M. Postnatal maturation of cochlear sensory hairs in the mouse. Anat Embryol (Berl) 1983;166:355–368. - PubMed
-
- Barbaric I, Wells S, Russ A, Dear TN. Spectrum of ENU-induced mutations in phenotype-driven and gene-driven screens in the mouse. Environ Mol Mutagen. 2007;48:124–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

