CTGF, intestinal stellate cells and carcinoid fibrogenesis
- PMID: 17876891
- PMCID: PMC4171302
- DOI: 10.3748/wjg.v13.i39.5208
CTGF, intestinal stellate cells and carcinoid fibrogenesis
Abstract
Aim: To investigate the role of small intestinal carcinoid tumor-derived fibrotic mediators, TGFbeta1 and CTGF, in the mediation of fibrosis via activation of an "intestinal" stellate cell.
Methods: GI carcinoid tumors were collected for Q RT-PCR analysis of CTGF and TGFbeta1. Markers of stellate cell desmoplasia were identified in peritoneal fibrosis by immunohistochemistry and stellate cells cultured from fresh resected fibrotic tissue. CTGF and TGFbeta1 were evaluated using quantitative tissue array profiling (AQUA analysis) in a GI carcinoid tissue microarray (TMA) with immunostaining and correlated with clinical and histologically documented fibrosis. Serum CTGF was analyzed using a sandwich ELISA assay.
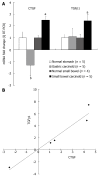
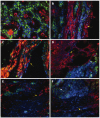
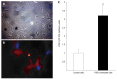
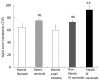
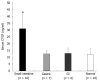
Results: Message levels of both CTGF and TGFbeta1 in SI carcinoid tumors were significantly increased (> 2-fold, P < 0.05) versus normal mucosa and gastric (non-fibrotic) carcinoids. Activated stellate cells and markers of stellate cell-mediated fibrosis (vimentin, desmin) were identified in histological fibrosis. An intestinal stellate cell was immunocytochemically and biochemically characterized and its TGFbeta1 (10-7M) initiated CTGF transcription response (> 3-fold, P < 0.05) demonstrated. In SI carcinoid tumor patients with documented fibrosis, TMA analysis demonstrated higher CTGF immunostaining (AQUA Score: 92 +/- 8; P < 0.05), as well as elevated TGFbeta1 (90.6 +/- 4.4, P < 0.05). Plasma CTGF (normal 12.5 +/- 2.6 ng/mL) was increased in SI carcinoid tumor patients (31 +/- 10 ng/mL, P < 0.05) compared to non-fibrotic GI carcinoids (< 15 ng/mL).
Conclusion: SI carcinoid tumor fibrosis is a CTGF/TGFbeta1-mediated stellate cell-driven fibrotic response. The delineation of the biology of fibrosis will facilitate diagnosis and enable development of agents to obviate its local and systemic complications.
Figures

References
-
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. - PubMed
-
- Modlin IM, Champaneria MC, Chan AK, Kidd M. A three-decade analysis of 3,911 small intestinal neuroendocrine tumors: the rapid pace of no progress. Am J Gastroenterol. 2007;102:1464–1473. - PubMed
-
- McNeal JE. Mechanism of obstruction in carcinoid tumors of the small intestine. Am J Clin Pathol. 1971;56:452–458. - PubMed
-
- Levy AD, Rimola J, Mehrotra AK, Sobin LH. From the archives of the AFIP: benign fibrous tumors and tumorlike lesions of the mesentery: radiologic-pathologic correlation. Radiographics. 2006;26:245–264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

