Stereospecific synthesis and characterization of oligodeoxyribonucleotides containing an N2-(1-carboxyethyl)-2'-deoxyguanosine
- PMID: 17877341
- PMCID: PMC3169888
- DOI: 10.1021/ja072130e
Stereospecific synthesis and characterization of oligodeoxyribonucleotides containing an N2-(1-carboxyethyl)-2'-deoxyguanosine
Abstract
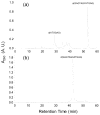
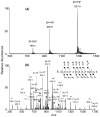
Methylglyoxal is a highly reactive alpha-ketoaldehyde that is produced endogenously and present in the environment and foods. It can modify DNA and proteins to form advanced glycation end products (AGEs). Emerging evidence has shown that N2-(1-carboxyethyl)-2'-deoxyguanosine (N2-CEdG) is a major marker for AGE-linked DNA adducts. Here, we report, for the first time, the preparation of oligodeoxyribonucleotides (ODNs) containing individual diastereomers of N2-CEdG via a postoligomerization synthesis method, which provided authentic substrates for examining the replication and repair of this lesion. In addition, thermodynamic parameters derived from melting temperature data revealed that the two diastereomers of N2-CEdG destabilized significantly the double helix as represented by a 4 kcal/mol increase in Gibbs free energy for duplex formation at 25 degrees C. Primer extension assay results demonstrated that both diastereomers of N2-CEdG could block considerably the replication synthesis mediated by the exonuclease-free Klenow fragment of Escherichia coli DNA polymerase I. Strikingly, the polymerase incorporated incorrect nucleotides, dGMP and dAMP, opposite the lesion more preferentially than the correct nucleotide, dCMP.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

