Simulated microgravity inhibits the proliferation and osteogenesis of rat bone marrow mesenchymal stem cells
- PMID: 17877609
- PMCID: PMC6496371
- DOI: 10.1111/j.1365-2184.2007.00461.x
Simulated microgravity inhibits the proliferation and osteogenesis of rat bone marrow mesenchymal stem cells
Erratum in
- Cell Prolif. 2008 Apr;41(2):375
Abstract
Objectives: Microgravity is known to affect the differentiation of bone marrow mesenchymal stem cells (BMSCs). However, a few controversial findings have recently been reported with respect to the effects of microgravity on BMSC proliferation. Thus, we investigated the effects of simulated microgravity on rat BMSC (rBMSC) proliferation and their osteogeneic potential.
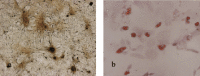
Materials and methods: rBMSCs isolated from marrow using our established effective method, based on erythrocyte lysis, were identified by their surface markers and their proliferation characteristics under normal conditions. Then, they were cultured in a clinostat to simulate microgravity, with or without growth factors, and in osteogenic medium. Subsequently, proliferation and cell cycle parameters were assessed using methylene blue staining and flow cytometry, respectively; gene expression was determined using Western blotting and microarray analysis.
Results: Simulated microgravity inhibited population growth of the rBMSCs, cells being arrested in the G(0)/G(1) phase of cell cycle. Growth factors, such as insulin-like growth factor-I, epidermal growth factor and basic fibroblastic growth factor, markedly stimulated rBMSC proliferation in normal gravity, but had only a slight effect in simulated microgravity. Akt and extracellular signal-related kinase 1/2 phosphorylation levels and the expression of core-binding factor alpha1 decreased after 3 days of clinorotation culture. Microarray and gene ontology analyses further confirmed that rBMSC proliferation and osteogenesis decreased under simulated microgravity.
Conclusions: The above data suggest that simulated microgravity inhibits population growth of rBMSCs and their differentiation towards osteoblasts. These changes may be responsible for some of the physiological changes noted during spaceflight.
Figures




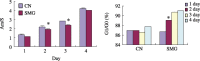


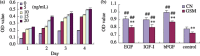
Similar articles
-
Simulated microgravity facilitates cell migration and neuroprotection after bone marrow stromal cell transplantation in spinal cord injury.Stem Cell Res Ther. 2013 Apr 1;4(2):35. doi: 10.1186/scrt184. Stem Cell Res Ther. 2013. PMID: 23548163 Free PMC article.
-
Effects of simulated microgravity on the expression profiles of RNA during osteogenic differentiation of human bone marrow mesenchymal stem cells.Cell Prolif. 2019 Mar;52(2):e12539. doi: 10.1111/cpr.12539. Epub 2018 Nov 5. Cell Prolif. 2019. PMID: 30397970 Free PMC article.
-
Maintenance of Neurogenic Differentiation Potential in Passaged Bone Marrow-Derived Human Mesenchymal Stem Cells Under Simulated Microgravity Conditions.Stem Cells Dev. 2019 Dec 1;28(23):1552-1561. doi: 10.1089/scd.2019.0146. Epub 2019 Nov 11. Stem Cells Dev. 2019. PMID: 31588849
-
The impact of simulated and real microgravity on bone cells and mesenchymal stem cells.Biomed Res Int. 2014;2014:928507. doi: 10.1155/2014/928507. Epub 2014 Jul 10. Biomed Res Int. 2014. PMID: 25110709 Free PMC article. Review.
-
Stem Cells toward the Future: The Space Challenge.Life (Basel). 2014 May 30;4(2):267-80. doi: 10.3390/life4020267. Life (Basel). 2014. PMID: 25370198 Free PMC article. Review.
Cited by
-
The individual and combined effects of spaceflight radiation and microgravity on biologic systems and functional outcomes.J Environ Sci Health C Toxicol Carcinog. 2021;39(2):129-179. doi: 10.1080/26896583.2021.1885283. J Environ Sci Health C Toxicol Carcinog. 2021. PMID: 33902391 Free PMC article.
-
Cell mechanosensitivity: mechanical properties and interaction with gravitational field.Biomed Res Int. 2013;2013:598461. doi: 10.1155/2013/598461. Epub 2012 Dec 26. Biomed Res Int. 2013. PMID: 23509748 Free PMC article. Review.
-
Real and Simulated Microgravity: Focus on Mammalian Extracellular Matrix.Life (Basel). 2022 Aug 29;12(9):1343. doi: 10.3390/life12091343. Life (Basel). 2022. PMID: 36143379 Free PMC article. Review.
-
Actin microfilament mediates osteoblast Cbfa1 responsiveness to BMP2 under simulated microgravity.PLoS One. 2013 May 10;8(5):e63661. doi: 10.1371/journal.pone.0063661. Print 2013. PLoS One. 2013. PMID: 23675497 Free PMC article.
-
Single Cell in a Gravity Field.Life (Basel). 2022 Oct 14;12(10):1601. doi: 10.3390/life12101601. Life (Basel). 2022. PMID: 36295035 Free PMC article. Review.
References
-
- Baker J, Liu JP, Robertson EJ, Efstratiadis A (1993) Role of insulin‐like growth factors in embryonic and postnatal growth. Cell 75, 73–82. - PubMed
-
- Basso N, Bellows CG, Heersche JN (2005) Effect of simulated weightlessness on osteoprogenitor cell number and proliferation in young and adult rats. Bone 36, 173–183. - PubMed
-
- Carmeliet G, Nys G, Bouillon R (1997) Microgravity reduces the differentiation of human osteoblastic MG‐63 cells. J. Bone Miner. Res. 12, 786–794. - PubMed
-
- Chen X, Xu H, Wan C, McCaigue M, Li G (2006) Bioreactor expansion of human adult bone marrow‐mesenchymal stem cells. Stem Cells 24, 2052–2059. - PubMed
-
- Colvin GA, Lambert JF, Carlson JE, McAuliffe CI, Abedi M, Quesenberry PJ (2002) Rhythmicity of engraftment and altered cell cycle kinetics of cytokine‐cultured murine marrow in simulated microgravity compared with static cultures. In Vitro Cell. Dev. Biol. Anim. 38, 343–351. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

