Skin keratinocytes pre-treated with embryonic stem cell-conditioned medium or BMP4 can be directed to an alternative cell lineage
- PMID: 17877610
- PMCID: PMC6496164
- DOI: 10.1111/j.1365-2184.2007.00464.x
Skin keratinocytes pre-treated with embryonic stem cell-conditioned medium or BMP4 can be directed to an alternative cell lineage
Abstract
Objectives: In this study, we have investigated whether secreted factors from embryonic stem cells (ESCs) could reprogramme keratinocytes and increase their potential to be directed into alternative cell lineages.
Materials and methods: Contact and non-contact co-cultures of skin keratinocytes and murine ESCs were used initially to confirm any reprogramming ability of ESC-conditioned medium (CM). Immunofluoresence was used to assess nuclear expression of octamer-4 (Oct-4), as well as to confirm neuronal protein expression in neuroectodermally directed keratinocytes. Transcript expression changes were evaluated using semiquantitative reverse transcription-polymerase chain reaction. Western blotting, accompanied by densitometry analysis, was used to evaluate protein expression following morphology changes.
Results: We found that keratinocytes treated with ESC-CM changed their morphology and were stimulated to express the pluripotency regulator, Oct-4, and its target transcripts, Sox-2, Nanog, Utf1 and Rex-1. We demonstrate that at least one of the reprogramming factors is bone morphogenetic factor-4 (BMP4). Pre-treated keratinocytes could be specifically directed to differentiate into cells of the neuronal lineage. The majority of responsive keratinocytes were the epidermal stem cell population, with a small percentage of transit-amplifying cells also being affected.
Conclusions: Our results suggest that ESC-CM contains a number of factors, including BMP4, which are capable of reprogramming mouse skin keratinocytes to make them more developmentally potent, as evidenced by their ability to be re-differentiated into cells of the neuronal lineage. Our findings also imply a continuum of differentiation within the basal keratinocyte population. An increase in developmental potential combined with directed differentiation could increase the therapeutic relevancy of somatic cells.
Figures







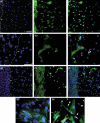
Similar articles
-
De-differentiation of mouse interfollicular keratinocytes by the embryonic transcription factor Oct-4.J Invest Dermatol. 2007 Feb;127(2):372-80. doi: 10.1038/sj.jid.5700531. Epub 2006 Aug 24. J Invest Dermatol. 2007. PMID: 16932739
-
Directed differentiation of human embryonic stem cells into keratinocyte progenitors in vitro: an attempt with promise of clinical use.In Vitro Cell Dev Biol Anim. 2016 Sep;52(8):885-93. doi: 10.1007/s11626-016-0024-2. Epub 2016 Aug 5. In Vitro Cell Dev Biol Anim. 2016. PMID: 27496193
-
Bone morphogenetic protein-2 (BMP-2) transactivates Dlx3 through Smad1 and Smad4: alternative mode for Dlx3 induction in mouse keratinocytes.Nucleic Acids Res. 2002 Jan 15;30(2):515-22. doi: 10.1093/nar/30.2.515. Nucleic Acids Res. 2002. PMID: 11788714 Free PMC article.
-
Single-stranded DNA binding protein Ssbp3 induces differentiation of mouse embryonic stem cells into trophoblast-like cells.Stem Cell Res Ther. 2016 May 28;7(1):79. doi: 10.1186/s13287-016-0340-1. Stem Cell Res Ther. 2016. PMID: 27236334 Free PMC article.
-
Intrinsic patterns of behavior of epithelial stem cells.J Investig Dermatol Symp Proc. 2004 Sep;9(3):208-14. doi: 10.1111/j.1087-0024.2004.09310.x. J Investig Dermatol Symp Proc. 2004. PMID: 15369215 Review.
Cited by
-
Characterization of a unique technique for culturing primary adult human epithelial progenitor/"stem cells".BMC Dermatol. 2012 Jun 24;12:8. doi: 10.1186/1471-5945-12-8. BMC Dermatol. 2012. PMID: 22726819 Free PMC article.
-
Three clonal types of urothelium with different capacities for replication.Cell Prolif. 2009 Dec;42(6):770-9. doi: 10.1111/j.1365-2184.2009.00647.x. Epub 2009 Sep 17. Cell Prolif. 2009. PMID: 19765021 Free PMC article.
-
Embryonic stem cell factors undifferentiated transcription factor-1 (UFT-1) and reduced expression protein-1 (REX-1) are widely expressed in human skin and may be involved in cutaneous differentiation but not in stem cell fate determination.Int J Exp Pathol. 2011 Oct;92(5):326-32. doi: 10.1111/j.1365-2613.2011.00769.x. Epub 2011 Mar 29. Int J Exp Pathol. 2011. PMID: 21446939 Free PMC article.
-
Gene expression signatures of extracellular matrix and growth factors during embryonic stem cell differentiation.PLoS One. 2012;7(10):e42580. doi: 10.1371/journal.pone.0042580. Epub 2012 Oct 15. PLoS One. 2012. PMID: 23077480 Free PMC article.
-
Human skin keratinocytes can be reprogrammed to express neuronal genes and proteins after a single treatment with decitabine.Biores Open Access. 2013 Jun;2(3):217-21. doi: 10.1089/biores.2012.0298. Biores Open Access. 2013. PMID: 23741634 Free PMC article.
References
-
- Byrne JA, Simonsson S, Western PS, Gurdon JB (2003) Nuclei of adult mammalian somatic cells are directly reprogrammed to oct‐4 stem cell gene expression by amphibian oocytes. Curr. Biol. 13, 1206–1213. - PubMed
-
- Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Karlstrom H, Lendahl U, Frisen J (2000) Generalized potential of adult neural stem cells. Science 288, 1660–1663. - PubMed
-
- Collas P, Taranger CK, Boquest AC, Noer A, Dahl JA (2006) On the way to reprogramming cells to pluripotency using cell‐free extracts. Reprod. Biomed. Online 12, 762–770. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

