The in vitro behaviour and patterns of colony formation of murine epithelial stem cells
- PMID: 17877611
- PMCID: PMC6496497
- DOI: 10.1111/j.1365-2184.2007.00467.x
The in vitro behaviour and patterns of colony formation of murine epithelial stem cells
Abstract
Objective: The mechanisms of renewal of skin and mucosal epithelia in vivo are associated with hierarchies of stem and amplifying cells organized in distinct spatial patterns. Stem and amplifying characteristics persist after isolation and growth of human keratinocytes in vitro but the pattern for murine keratinocytes has been less clear.
Materials and methods: Murine keratinocytes were grown in low calcium media and examined for their patterns of colony morphologies.
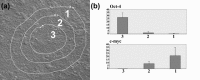
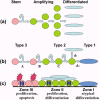
Results: We consistently identified three types of colonies, one of which contains concentric zones of amplifying and differentiated cells surrounding a central zone of cells that have patterns of expression and behavioural characteristic of stem cells. This zonal organization facilitated analysis of stem cell formation and loss. Cells in the central stem cell zone undergo rapid symmetric divisions but expansion of this population is partially limited by their peripheral transition into amplifying cells. A striking feature of central zone cells is their enhanced apoptotic susceptibility and stem cell expansion limited by consistently high background rates of apoptosis. This occasionally reaches catastrophic levels with elimination of the entire central zone.
Conclusion: In vitro amplification of stem cells for the generation of engineered tissue has tended to focus on control of asymmetric division but these findings suggest that development of mechanisms protecting stem cells from apoptotic changes are also likely to be of particular value.
Figures



References
-
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T (2004) Tie2/angiopoietin‐1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118, 149–161. - PubMed
-
- Bickenbach JR (1981) Identification and behavior of label‐retaining cells in oral mucosa and skin. J. Dent. Res. 60, 1611–1620. - PubMed
-
- Bickenbach JR, Chism E (1998) Selection and extended growth of murine epidermal stem cells in culture. Exp. Cell Res. 244, 184–195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

