Cyclin-dependent kinase inhibitors and basement membrane interact to regulate breast epithelial cell differentiation and acinar morphogenesis
- PMID: 17877612
- PMCID: PMC6496798
- DOI: 10.1111/j.1365-2184.2007.00463.x
Cyclin-dependent kinase inhibitors and basement membrane interact to regulate breast epithelial cell differentiation and acinar morphogenesis
Abstract
Objective: The cyclin-dependent kinase inhibitors (CDKIs), p21(CIP1) and p27(KIP1) regulate growth and differentiation in diverse tissue types. We aimed to determine whether p21(CIP1) or p27(KIP1) could induce a terminally differentiated phenotype in breast cells, and to examine if CDKI expression is regulated by basement membrane interactions.
Materials and methods: Effects of increased CDKI expression on the phenotype of MCF-10A breast epithelial cells were examined by retroviral transduction of p21(CIP1) or p27(KIP1) cDNA.
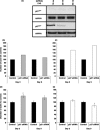
Results: Overexpression of p21(CIP1) or p27(KIP1) reduced MCF-10A growth rates in monolayer cultures, altered cellular morphology and stimulated accumulation of neutral lipid droplets, suggesting partial lactational differentiation. However, markers of luminal differentiation (oestrogen and progesterone receptors, alpha-lactalbumin, beta-casein and adipophilin) were absent when examined by reverse transcriptase-polymerase chain reaction and immunohistochemistry. Cell-basement membrane contacts are known to be essential for full mammary epithelial cell differentiation and therefore parental MCF-10A cells were cultured on a basement membrane preparation (Matrigel) in which they form acini. Immunocytochemistry showed that Ki67, the cell proliferation marker, was initially expressed at high levels and as growth decreased p27(KIP1) expression steadily increased. Surprisingly, p21(CIP1) was highest at the early stages of acinus growth and was detected in proliferating cells, as demonstrated by colocalization in dual Ki67/p21(CIP1) immunofluorescence. Overexpression of p21(CIP1) or p27(KIP1) impaired formation of acini, whereas their knockdown, using siRNA, increased acinus formation.
Conclusion: We conclude that both p21(CIP1) and p27(KIP1) induce partial secretory differentiation of mammary cells in monolayer, but during acinus morphogenesis in 3D culture they have a highly regulated temporal expression pattern.
Figures
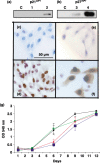
 ) and p27KIP1 (
) and p27KIP1 ( ) transduced cells showed retarded growth compared to control cells (
) transduced cells showed retarded growth compared to control cells ( ) or vector treated cells (
) or vector treated cells ( ). Results are expressed as average ± SEM.
). Results are expressed as average ± SEM.
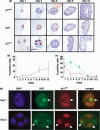
 ) (b), Ki67 (
) (b), Ki67 ( ) and p21CIP1 (
) and p21CIP1 ( ) (c). (d) Representative photomicrographs showing colocalization of Ki67 and p21CIP1 MCF‐10A cells were cultured on Matrigel, fixed and sectioned as described in Materials and Methods. Sections were subjected to dual immunofluorescence for p21CIP1 (red) and Ki67 (green). Nuclei were counterstained with DAPI (blue). Dual‐labelled cells are indicated by arrows.
) (c). (d) Representative photomicrographs showing colocalization of Ki67 and p21CIP1 MCF‐10A cells were cultured on Matrigel, fixed and sectioned as described in Materials and Methods. Sections were subjected to dual immunofluorescence for p21CIP1 (red) and Ki67 (green). Nuclei were counterstained with DAPI (blue). Dual‐labelled cells are indicated by arrows.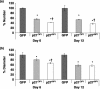
References
-
- Aggeler J, Ward J, Blackie LM, Barcellos‐Hoff MH, Streuli CH, Bissell MJ (1991) Cytodifferentiation of mouse mammary epithelial cells cultured on a reconstituted basement membrane reveals striking similarities to development in vivo . J. Cell Sci. 99, 407–417. - PubMed
-
- Anderson E, Clarke RB, Howell A (1998) Estrogen responsiveness and control of normal human breast proliferation. J. Mammary Gland Biol. Neoplasia 3, 23–35. - PubMed
-
- Buckley S, Driscoll B, Anderson KD, Warburton D (1997) Cell cycle in alveolar epithelial type II cells: integration of Matrigel and KGF. Am. J. Physiol. 273, L572–L580. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

