Once-daily OROS hydromorphone for the management of chronic nonmalignant pain: a dose-conversion and titration study
- PMID: 17877652
- PMCID: PMC2040191
- DOI: 10.1111/j.1742-1241.2007.01500.x
Once-daily OROS hydromorphone for the management of chronic nonmalignant pain: a dose-conversion and titration study
Abstract
Background: The use of opioid analgesics for patients with chronic nonmalignant pain is becoming more widely accepted, and long-acting formulations are an important treatment option.
Aim: To assess conversion to extended-release OROS hydromorphone from previous stable opioid agonist therapy in patients with chronic nonmalignant pain of moderate-to-severe intensity.
Methods: In this open-label multicentre trial, patients were stabilised on their previous opioid therapy before being switched to OROS hydromorphone at a ratio of 5 : 1 (morphine sulphate equivalent to hydromorphone hydrochloride). The OROS hydromorphone dose was titrated over 3-16 days to achieve effective analgesia, and maintenance treatment continued for 14 days.
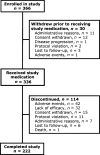
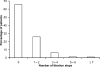
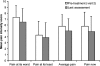
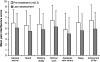
Results: Study medication was received by 336 patients; 66% completed all study phases. Stabilisation of OROS hydromorphone was achieved by 94.6% of patients, the majority in two or fewer titration steps (mean time, 4.2 days). Mean pain intensity scores, as determined by the Brief Pain Inventory, decreased during OROS hydromorphone treatment (p <or= 0.001). The percentage of patients rating their pain relief as 'good' or 'complete' increased, and the use of rescue analgesics for breakthrough pain decreased. The interference of pain with everyday activities (e.g. walking or work), and the effects on mood and enjoyment of life, also improved during the study (all p < 0.001). OROS hydromorphone was well tolerated, and adverse events were those expected for opioid agonist therapy.
Conclusion: Patients with chronic nonmalignant pain who had been receiving opioid therapy easily underwent conversion to OROS hydromorphone, with no loss of efficacy or increase in adverse events.
Trial registration: ClinicalTrials.gov NCT00410644.
Figures
References
-
- Passik SD, Weinreb HJ. Managing chronic nonmalignant pain: overcoming obstacles to the use of opioids. Adv Ther. 2000;17:70–83. - PubMed
-
- Breivik H. Opioids in chronic non-cancer pain, indications and controversies. Eur J Pain. 2005;9:127–30. - PubMed
-
- Sarhill N, Walsh D, Nelson KA. Hydromorphone: pharmacology and clinical applications in cancer patients. Support Care Cancer. 2001;9:84–96. - PubMed
-
- Vallner JJ, Stewart JT, Kotzan JA, et al. Pharmacokinetics and bioavailability of hydromorphone following intravenous and oral administration to human subjects. J Clin Pharmacol. 1981;21:152–56. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

