A fatal case of bupropion (Zyban) hepatotoxicity with autoimmune features: Case report
- PMID: 17877816
- PMCID: PMC2008202
- DOI: 10.1186/1752-1947-1-88
A fatal case of bupropion (Zyban) hepatotoxicity with autoimmune features: Case report
Abstract
Background: Bupropion is approved for the treatment of mood disorders and as an adjuvant medication for smoking cessation. Bupropion is generally well tolerated and considered safe. Two randomized controlled trials of bupropion therapy for smoking cessation did not report any hepatic adverse events. However, there are three reports of severe but non-fatal bupropion hepatotoxicity published in the literature.
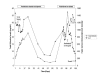
Case presentation: We present the case of a 55-year old man who presented with jaundice and severe hepatic injury approximately 6 months after starting bupropion for smoking cessation. Laboratory evaluation demonstrated a mixed picture of hepatocellular injury and cholestasis. Liver biopsy demonstrated findings consistent with severe hepatotoxic injury due to drug induced liver injury. Laboratory testing was also notable for positive autoimmune markers. The patient initially had clinical improvement with steroid therapy but eventually died of infectious complications.
Conclusion: This report represents the first fatal report of bupropion related hepatotoxicity and the second case of bupropion related liver injury demonstrating autoimmune features. The common use of this medication for multiple indications makes it important for physicians to consider this medication as an etiologic agent in patients with otherwise unexplained hepatocellular jaundice.
Figures

Similar articles
-
Rapid bupropion-induced hepatotoxicity: a case report and review of the literature.J Med Case Rep. 2018 Feb 24;12(1):46. doi: 10.1186/s13256-018-1563-9. J Med Case Rep. 2018. PMID: 29475455 Free PMC article.
-
Acute liver failure with concurrent bupropion and carbimazole therapy.Ann Pharmacother. 2003 Feb;37(2):220-3. doi: 10.1177/106002800303700212. Ann Pharmacother. 2003. PMID: 12549952
-
[Bupropion SR for weaning from smoking in relapsed smokers: results of an open multicentre trial in Germany].Pneumologie. 2004 Mar;58(3):140-6. doi: 10.1055/s-2004-818377. Pneumologie. 2004. PMID: 15007784 Clinical Trial. German.
-
Review of bupropion for smoking cessation.Drug Alcohol Rev. 2003 Jun;22(2):203-20. doi: 10.1080/09595230100100642. Drug Alcohol Rev. 2003. PMID: 12850907 Review.
-
[Bupropion: an effective new aid for smoking cessation].Ned Tijdschr Geneeskd. 2000 Nov 4;144(45):2138-42. Ned Tijdschr Geneeskd. 2000. PMID: 11086486 Review. Dutch.
Cited by
-
Psychotropic drugs and liver disease: A critical review of pharmacokinetics and liver toxicity.World J Gastrointest Pharmacol Ther. 2017 Feb 6;8(1):26-38. doi: 10.4292/wjgpt.v8.i1.26. World J Gastrointest Pharmacol Ther. 2017. PMID: 28217372 Free PMC article. Review.
-
Acute Hepatocellular Drug-Induced Liver Injury From Bupropion and Doxycycline.ACG Case Rep J. 2015 Oct 9;3(1):66-8. doi: 10.14309/crj.2015.103. eCollection 2015 Oct. ACG Case Rep J. 2015. PMID: 26504884 Free PMC article.
-
Practical guidelines for diagnosis and early management of drug-induced liver injury.World J Gastroenterol. 2008 Nov 28;14(44):6774-85. doi: 10.3748/wjg.14.6774. World J Gastroenterol. 2008. PMID: 19058303 Free PMC article. Review.
-
Rapid bupropion-induced hepatotoxicity: a case report and review of the literature.J Med Case Rep. 2018 Feb 24;12(1):46. doi: 10.1186/s13256-018-1563-9. J Med Case Rep. 2018. PMID: 29475455 Free PMC article.
-
A consensus statement for safety monitoring guidelines of treatments for major depressive disorder.Aust N Z J Psychiatry. 2011 Sep;45(9):712-25. doi: 10.3109/00048674.2011.595686. Aust N Z J Psychiatry. 2011. PMID: 21888608 Free PMC article. Review.
References
-
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Smith SS, Muramoto ML, Daughton DM, Doan K, Fiore MC, Baker TB. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med. 1999;340:685–91. - PubMed
-
- Oslin DW, Duffy K. The rise of serum aminotransferases in a patient treated with bupropion. J Clin Psychopharmacol. 1993;13:364–5. - PubMed
-
- Hu KQ, Tiyyagura L, Kanel G, Redeker AG. Acute hepatitis induced by bupropion. Dig Dis Sci. 2000;45:1872–3. - PubMed
-
- Alvaro D, Onetti-Muda A, Moscatelli R, Attili AF. Acute cholestatic hepatitis induced by bupropion prescribed as pharmacological support to stop smoking. A case report. Dig Liver Dis. 2001;33:703–6. - PubMed
-
- Tucker WE. Preclinical toxicology of bupropion: an overview. J Clin Psychiatry. 1983;44:60–2. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

