Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function
- PMID: 17878306
- PMCID: PMC2000503
- DOI: 10.1073/pnas.0705891104
Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function
Abstract
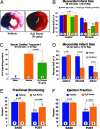
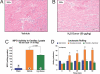
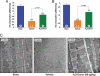
The recent discovery that hydrogen sulfide (H(2)S) is an endogenously produced gaseous second messenger capable of modulating many physiological processes, much like nitric oxide, prompted us to investigate the potential of H(2)S as a cardioprotective agent. In the current study, we demonstrate that the delivery of H(2)S at the time of reperfusion limits infarct size and preserves left ventricular (LV) function in an in vivo model of myocardial ischemia-reperfusion (MI-R). This observed cytoprotection is associated with an inhibition of myocardial inflammation and a preservation of both mitochondrial structure and function after I-R injury. Additionally, we show that modulation of endogenously produced H(2)S by cardiac-specific overexpression of cystathionine gamma-lyase (alpha-MHC-CGL-Tg mouse) significantly limits the extent of injury. These findings demonstrate that H(2)S may be of value in cytoprotection during the evolution of myocardial infarction and that either administration of H(2)S or the modulation of endogenous production may be of clinical benefit in ischemic disorders.
Conflict of interest statement
Conflict of interest statement: Funding for this study was provided by Ikaria, Inc. (Seattle, WA), and the hydrogen sulfide donor was also provided by Ikaria. D.J.L. is currently serving as a paid consultant to Ikaria, and C.S. is currently employed by Ikaria.
Figures

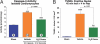
References
-
- Beauchamp RO, Jr, Bus JS, Popp JA, Boreiko CJ, Andjelkovich DA. Crit Rev Toxicol. 1984;13:25–97. - PubMed
-
- Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, Squadrito GL, Davies KJ. Am J Physiol. 2006;291:R491–R511. - PubMed
-
- Kimura H. Biochem Biophys Res Commun. 2000;267:129–133. - PubMed
-
- Boehning D, Snyder SH. Annu Rev Neurosci. 2003;26:105–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

