Principal component analysis of the pH-dependent conformational transitions of bovine beta-lactoglobulin monitored by heteronuclear NMR
- PMID: 17878316
- PMCID: PMC2000507
- DOI: 10.1073/pnas.0702112104
Principal component analysis of the pH-dependent conformational transitions of bovine beta-lactoglobulin monitored by heteronuclear NMR
Abstract
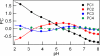
To clarify the pH-dependent conformational transitions of proteins, we propose an approach in which structural changes monitored by heteronuclear sequential quantum correlation (HSQC) spectroscopy were analyzed by using a principal component analysis (PCA). We use bovine beta-lactoglobulin, a protein widely used in protein folding studies, as a target. First, we measured HSQC spectra at various pH values and subjected them to a PCA. The analysis revealed three apparent transitions with pK(a) values of 2.9, 4.9, and 6.8, consistent with previous reports using different methods. Next, Gdn-HCl-induced unfolding was examined by measuring tryptophan fluorescence at various pH values. Between pH 2 and 8, beta-lactoglobulin exhibited a number of structural transitions as well as changes in stability represented by the free energy change of unfolding, DeltaG(U). By combining the NMR and fluorescence results, the change in DeltaG(U) was suggested to result from the decreased pK(a) of some acidic residues. Notably, the native state at neutral pH is destabilized by deprotonation of Glu-89, leading to an increase in the relative population of the intermediate. Thus, the PCA of pH-dependent HSQC spectra provides a more comprehensive understanding of the stability and function of proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
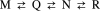



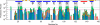
Similar articles
-
Conformation and stability of thiol-modified bovine beta-lactoglobulin.Protein Sci. 2000 Sep;9(9):1719-29. Protein Sci. 2000. PMID: 11045618 Free PMC article.
-
Unfolding and refolding of bovine beta-lactoglobulin monitored by hydrogen exchange measurements.J Mol Biol. 1999 Nov 5;293(4):953-69. doi: 10.1006/jmbi.1999.3191. J Mol Biol. 1999. PMID: 10543977
-
Pressure denaturation of beta-lactoglobulin. Different stabilities of isoforms A and B, and an investigation of the Tanford transition.Eur J Biochem. 2000 Apr;267(8):2235-41. doi: 10.1046/j.1432-1327.2000.01226.x. Eur J Biochem. 2000. PMID: 10759846
-
Structural dynamics and folding of beta-lactoglobulin probed by heteronuclear NMR.Biochim Biophys Acta. 2009 Jun;1790(6):527-37. doi: 10.1016/j.bbagen.2009.04.003. Epub 2009 Apr 10. Biochim Biophys Acta. 2009. PMID: 19362581 Review.
-
Thermal unfolding of beta-lactoglobulin, alpha-lactalbumin, and bovine serum albumin. A thermodynamic approach.Crit Rev Food Sci Nutr. 1996 Jul;36(6):565-601. doi: 10.1080/10408399609527740. Crit Rev Food Sci Nutr. 1996. PMID: 8841732 Review.
Cited by
-
Binding Isotherms and Time Courses Readily from Magnetic Resonance.Anal Chem. 2016 Aug 16;88(16):8172-8. doi: 10.1021/acs.analchem.6b01918. Epub 2016 Aug 5. Anal Chem. 2016. PMID: 27458657 Free PMC article.
-
Protein dielectric constants determined from NMR chemical shift perturbations.J Am Chem Soc. 2013 Nov 13;135(45):16968-76. doi: 10.1021/ja406995j. Epub 2013 Oct 31. J Am Chem Soc. 2013. PMID: 24124752 Free PMC article.
-
Applications of NMR and computational methodologies to study protein dynamics.Arch Biochem Biophys. 2017 Aug 15;628:71-80. doi: 10.1016/j.abb.2017.05.002. Epub 2017 May 5. Arch Biochem Biophys. 2017. PMID: 28483383 Free PMC article. Review.
-
Engineered β-Lactoglobulin Produced in E. coli: Purification, Biophysical and Structural Characterisation.Mol Biotechnol. 2016 Oct;58(10):605-618. doi: 10.1007/s12033-016-9960-z. Mol Biotechnol. 2016. PMID: 27380951 Free PMC article.
-
Fast mapping of global protein folding states by multivariate NMR: a GPS for proteins.PLoS One. 2010 Apr 21;5(4):e10262. doi: 10.1371/journal.pone.0010262. PLoS One. 2010. PMID: 20421996 Free PMC article.
References
-
- Jaumot J, Vives M, Gargallo R. Anal Biochem. 2004;327:1–13. - PubMed
-
- Zhou Z, Madrid M, Evanseck JD, Madura JD. J Am Chem Soc. 2005;127:17253–17260. - PubMed
-
- Jaumot J, Marchán V, Gargallo R, Grandas A, Tauler R. Anal Chem. 2004;76:7094–7101. - PubMed
-
- Szundi I, Liao GL, Einarsdóttir Ó. Biochemistry. 2001;40:2332–2339. - PubMed
-
- Tanford C. Adv Protein Chem. 1970;24:1–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

