Melanosomes--dark organelles enlighten endosomal membrane transport
- PMID: 17878918
- PMCID: PMC2786984
- DOI: 10.1038/nrm2258
Melanosomes--dark organelles enlighten endosomal membrane transport
Abstract
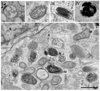
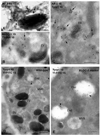
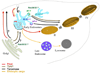
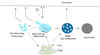
Melanosomes are tissue-specific lysosome-related organelles of pigment cells in which melanins are synthesized and stored. Analyses of the trafficking and fate of melanosomal components are beginning to reveal how melanosomes are formed through novel pathways from early endosomal intermediates. These studies unveil generalized structural and functional modifications of the endosomal system in specialized cells, and provide unexpected insights into the biogenesis of multivesicular bodies and how compartmentalization regulates protein refolding. Moreover, genetic disorders that affect the biogenesis of melanosomes and other lysosome-related organelles have shed light onto the molecular machinery that controls specialized endosomal sorting events.
Figures

References
-
- Gruenberg J. The endocytic pathway: a mosaic of domains. Nature Reviews, Mol. Cell Biol. 2001;2:721–730. - PubMed
-
- Maxfield FR, McGraw TE. Endocytic recycling. Nature Rev. Mol. Cell Biol. 2004;5:121–132. - PubMed
-
- Spritz RA, Chiang PW, Oiso N, Alkhateeb A. Human and mouse disorders of pigmentation. Curr. Opin. Genet. Dev. 2003;13:284–289. - PubMed
-
- Jeffery G. Architecture of the optic chiasm and the mechanisms that sculpt its development. Physiol. Rev. 2001;81:1393–1414. - PubMed
-
- Wakamatsu K, Ito S. Advanced chemical methods in melanin determination. Pigment Cell Res. 2002;15:162–173. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

