Cytometric assessment of DNA damage by exogenous and endogenous oxidants reports aging-related processes
- PMID: 17879239
- PMCID: PMC3860741
- DOI: 10.1002/cyto.a.20469
Cytometric assessment of DNA damage by exogenous and endogenous oxidants reports aging-related processes
Abstract
The ongoing DNA damage caused by reactive oxygen species generated during oxidative metabolism is considered a key factor contributing to cell aging as well as preconditioning cells to neoplastic transformation. We postulated before that a significant fraction of constitutive histone H2AX phosphorylation (CHP) and constitutive activation of ATM (CAA) seen in untreated normal and tumor cells occurs in response to such DNA damage. In the present study, we provide further evidence in support of this postulate. The level of ATM activation and H2AX phosphorylation, detected immunocytochemically, has been monitored in WI-38, A549, and TK6 cells treated with H2O2 as well as growing under conditions known or suspected to affect the level of endogenous oxidants. Thirty- to 60-min exposure of cells to 100 or 200 microM H2O2 led to an increase in the level of H2AX phosphorylation and ATM activation, particularly pronounced (nearly fivefold) in S-phase cells. Cell growth for 24-48 h under hypoxic conditions (3% O2) distinctly lowered the level of CHP and CAA while it had minor effect on cell cycle progression. Treatment (4 h) with 0.1 or 0.3 mM 3-bromopyruvate, an inhibitor of glycolysis and mitochondrial oxidative phosphorylation, reduced the level of CHP (up to fourfold) and also decreased the level of CAA. Growth of WI-38 cells in 2% serum concentration for 48 h led to a 25 and 30% reduction in CHP and CHA, respectively, compared with cells growing in 10% serum. The antioxidant vitamin C (2 mM) reduced CHP and CAA by 20-30% after 24 h of treatment, while the COX-2 inhibitor celecoxib (5 microM) had a minor effect on CHP and CAA, though it decreased the level of H2O2-induced H2AX phosphorylation and ATM activation. In contrast, dichloroacetate known to shift metabolism from anaerobic to oxidative glycolysis through its effect on pyruvate dehydrogenase kinase enhanced the level of CHP and CAA. Our present data and earlier observations strongly support the postulate that a large fraction of CHP and CAA occurs in response to DNA damage caused by metabolically generated oxidants. Cytometric analysis of CHP and CAA provides the means to measure the effectiveness of exogenous factors, which either through lowering aerobic metabolism or neutralizing radicals may protect DNA from such damage.
Copyright (c) 2007 International Society for Analytical Cytology.
Figures
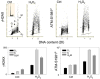

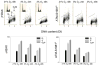
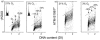
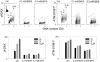
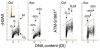
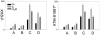
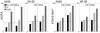
Comment in
-
Cytometric assessment of DNA damage: wondering between life and death.Cytometry A. 2007 Nov;71(11):899-900. doi: 10.1002/cyto.a.20477. Cytometry A. 2007. PMID: 17943996 No abstract available.
References
-
- Barzilai A, Yamamoto K. DNA damage responses to oxidative stress. DNA repair (Amst) 2004;3:1109–1115. - PubMed
-
- Nohl H. Generation of superoxide radicals as byproducts of cellular respiration. Ann Biol Clin. 1994;52:199–204. - PubMed
-
- Moller P, Loft S. Interventions with antioxidants and nutrients in relation to oxidative DNA damage and repair. Mutat Res. 2004;551:79–89. - PubMed
-
- Beckman KB, Ames BN. Oxidative decay of DNA. J Biol Chem. 1997;272:13300–13305. - PubMed
-
- Dianov GL, Parsons JL. Co-ordination of DNA single strand break repair. DNA repair (Amst) 2007;6:454–460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

