Sinomenine, a natural dextrorotatory morphinan analog, is anti-inflammatory and neuroprotective through inhibition of microglial NADPH oxidase
- PMID: 17880684
- PMCID: PMC2064906
- DOI: 10.1186/1742-2094-4-23
Sinomenine, a natural dextrorotatory morphinan analog, is anti-inflammatory and neuroprotective through inhibition of microglial NADPH oxidase
Abstract
Background: The mechanisms involved in the induction and regulation of inflammation resulting in dopaminergic (DA) neurotoxicity in Parkinson's disease (PD) are complex and incompletely understood. Microglia-mediated inflammation has recently been implicated as a critical mechanism responsible for progressive neurodegeneration.
Methods: Mesencephalic neuron-glia cultures and reconstituted cultures were used to investigate the molecular mechanisms of sinomenine (SN)-mediated anti-inflammatory and neuroprotective effects in both the lipopolysaccharide (LPS)- and the 1-methyl-4-phenylpyridinium (MPP+)-mediated models of PD.
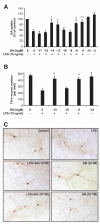
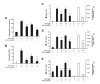
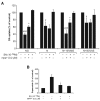
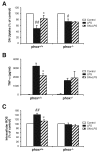
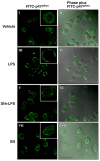
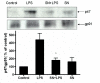
Results: SN showed equivalent efficacy in protecting against DA neuron death in rat midbrain neuron-glial cultures at both micro- and sub-picomolar concentrations, but no protection was seen at nanomolar concentrations. The neuroprotective effect of SN was attributed to inhibition of microglial activation, since SN significantly decreased tumor necrosis factor-alpha (TNF-alpha, prostaglandin E2 (PGE2) and reactive oxygen species (ROS) production by microglia. In addition, from the therapeutic point of view, we focused on sub-picomolar concentration of SN for further mechanistic studies. We found that 10(-14) M of SN failed to protect DA neurons against MPP+-induced toxicity in the absence of microglia. More importantly, SN failed to show a protective effect in neuron-glia cultures from mice lacking functional NADPH oxidase (PHOX), a key enzyme for extracellular superoxide production in immune cells. Furthermore, we demonstrated that SN reduced LPS-induced extracellular ROS production through the inhibition of the PHOX cytosolic subunit p47phoxtranslocation to the cell membrane.
Conclusion: Our findings strongly suggest that the protective effects of SN are most likely mediated through the inhibition of microglial PHOX activity. These findings suggest a novel therapy to treat inflammation-mediated neurodegenerative diseases.
Figures
References
-
- McGeer PL SI, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

