Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala
- PMID: 17880899
- PMCID: PMC2042139
- DOI: 10.1016/j.neuron.2007.08.004
Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala
Abstract
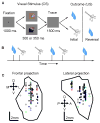
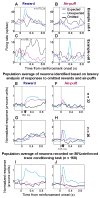
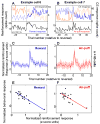
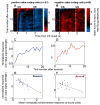
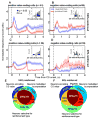
Animals and humans learn to approach and acquire pleasant stimuli and to avoid or defend against aversive ones. However, both pleasant and aversive stimuli can elicit arousal and attention, and their salience or intensity increases when they occur by surprise. Thus, adaptive behavior may require that neural circuits compute both stimulus valence--or value--and intensity. To explore how these computations may be implemented, we examined neural responses in the primate amygdala to unexpected reinforcement during learning. Many amygdala neurons responded differently to reinforcement depending upon whether or not it was expected. In some neurons, this modulation occurred only for rewards or aversive stimuli, but not both. In other neurons, expectation similarly modulated responses to both rewards and punishments. These different neuronal populations may subserve two sorts of processes mediated by the amygdala: those activated by surprising reinforcements of both valences-such as enhanced arousal and attention-and those that are valence-specific, such as fear or reward-seeking behavior.
Figures



References
-
- Amaral D, Price J, Pitkanen A, Carmichael S. Anatomical Organization of the Primate Amygdaloid Complex. In: Aggleton J, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. New York: Wiley-Liss, Inc; 1992. pp. 1–66.
-
- Anstrom KK, Woodward DJ. Restraint increases dopaminergic burst firing in awake rats. Neuropsychopharmacology. 2005;30:1832–1840. - PubMed
-
- Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends in Neurosciences. 2006;29:272–279. - PubMed
-
- Baxter M, Murray EA. The amygdala and reward. Nature Reviews Neuroscience. 2002;3:563–573. - PubMed
-
- Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton J, editor. The Amygdala: A Functional Analysis. New York: Oxford University Press; 2000. pp. 213–287.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

