Long-term treatment sequelae after external beam irradiation with or without hormonal manipulation for adenocarcinoma of the prostate: analysis of radiation therapy oncology group studies 85-31, 86-10, and 92-02
- PMID: 17881145
- PMCID: PMC2917176
- DOI: 10.1016/j.ijrobp.2007.06.050
Long-term treatment sequelae after external beam irradiation with or without hormonal manipulation for adenocarcinoma of the prostate: analysis of radiation therapy oncology group studies 85-31, 86-10, and 92-02
Abstract
Purpose: Late gastrointestinal (GI) and genitourinary (GU) morbidity from external beam irradiation used to treat adenocarcinoma of the prostate continue to be a concern of physicians and patients alike. In addition, for locally advanced/high-risk cancer, the appropriate use of hormonal manipulation in addition to radiation therapy (RT) may increase toxicity. We analyzed three large Radiation Therapy Oncology Group (RTOG) studies (85-31, 86-10, and 92-02) to try to address these issues.
Methods and materials: A total of 2,922 patients were accrued with a median follow-up of 10.3 years for surviving patients. The RTOG scoring scheme was used to assess GI, GU, and other toxicities. Toxicity reported was Grade 3 or higher late toxicity. Patient toxicity level was assessed by study and by treatment type combining RT only vs. RT + short-course hormone therapy (STH) vs. RT + long-term hormone therapy (LTH).
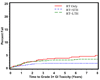
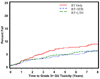
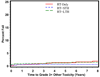
Results: Multivariate analysis reveals that age >70 was statistically significantly associated with a decrease in late any Grade 3+ toxicity (hazard ratio [HR] = 0.78, p = 0.0476) adjusted for treatment type. Comparing treatment type, patients treated with RT+STH had a statistically significant lower probability of Grade 3+ GI, GU, and other toxicity compared with RT alone (p = .00006; p = 0.0037; p = 0.0127, respectively). Patients treated with RT+LTH had a statistically significant lower probability of Grade 3+ GU toxicity compared with RT alone (p = 0.023).
Conclusions: These data show that external beam radiation therapy remains a safe option for locally advanced/high-risk prostate cancer, and the use of hormonal manipulation does appear to be protective for GU and GI toxicity depending upon length of treatment.
Conflict of interest statement
Conflict of Interest: None
Figures
References
-
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the RTOG and EORTC. Int J Rad Onc Biol Phys. 1995;31:1341–1346. - PubMed
-
- Lawton CA, Won M, Pilepich M, et al. Long-term treatment sequelae following external beam radiation for adenocarcinoma of the prostate: Analysis of RTOG studies 75-06 and 77-06. Int J Rad Onc Biol & Phys. 1991;21:935–939. - PubMed
-
- Pilepich M, Winter K, Lawton C, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma – long term results of phase III RTOG 85-31. Int J Rad Onc Biol & Phys. 2005;61(5):1285–1290. - PubMed
-
- Pilepich M, Winter K, John M, et al. Phase III RTOG trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Rad Onc Biol & Phys. 2001;50(5):1243–1252. - PubMed
-
- Hanks G, Pajak T, Porter A, et al. Phase III trial of long term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: RTOG protocol 92-02. J Clin Onc. 2003;21(21):3972–3978. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical