Induction of epidermal growth factor receptor expression by Epstein-Barr virus latent membrane protein 1 C-terminal-activating region 1 is mediated by NF-kappaB p50 homodimer/Bcl-3 complexes
- PMID: 17881446
- PMCID: PMC2169135
- DOI: 10.1128/JVI.01601-07
Induction of epidermal growth factor receptor expression by Epstein-Barr virus latent membrane protein 1 C-terminal-activating region 1 is mediated by NF-kappaB p50 homodimer/Bcl-3 complexes
Abstract
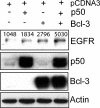
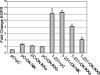
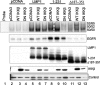
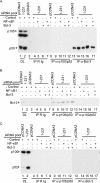
The Epstein-Barr virus (EBV) is associated with the development of numerous malignancies, including the epithelial malignancy nasopharyngeal carcinoma (NPC). The viral oncoprotein latent membrane protein 1 (LMP1) is expressed in almost all EBV-associated malignancies and has profound effects on gene expression. LMP1 acts as a constitutively active tumor necrosis factor receptor and activates multiple forms of the NF-kappaB family of transcription factors. LMP1 has two domains that both activate NF-kappaB. In epithelial cells, LMP1 C-terminal activating region 1 (CTAR1) uniquely activates p50/p50-, p50/p52-, and p65-containing complexes while CTAR2 activates canonical p50/p65 complexes. CTAR1 also uniquely upregulates the epidermal growth factor receptor (EGFR). In NPC, NF-kappaB p50/p50 homodimers and the transactivator Bcl-3 were detected on the EGFR promoter. In this study, the role of NF-kappaB p50 and Bcl-3 in LMP1-mediated upregulation of EGFR was analyzed. In LMP1-CTAR1-expressing cells, chromatin immunoprecipitation detected p50 and Bcl-3 on the NF-kappaB consensus sites within the egfr promoter. Transient overexpression of p50 and Bcl-3 increased EGFR expression, confirming the regulation of EGFR by these factors. Treatment with p105/p50 siRNA effectively reduced p105/p50 levels but unexpectedly increased Bcl-3 expression and levels of p50/Bcl-3 complexes, resulting in increased EGFR expression. These data suggest that induction of p50/p50/Bcl-3 complexes by LMP1 CTAR1 mediates LMP1-induced EGFR upregulation and that formation of the p50/p50/Bcl-3 complex is negatively regulated by the p105 precursor. The distinct forms of NF-kappaB that are induced by LMP1 CTAR1 likely activate distinct cellular genes.
Figures



Similar articles
-
Changes in expression induced by Epstein-Barr Virus LMP1-CTAR1: potential role of bcl3.mBio. 2015 Apr 14;6(2):e00441-15. doi: 10.1128/mBio.00441-15. mBio. 2015. PMID: 25873381 Free PMC article.
-
Epstein-Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor through effects on Bcl-3 and STAT3.J Virol. 2008 Jun;82(11):5486-93. doi: 10.1128/JVI.00125-08. Epub 2008 Mar 26. J Virol. 2008. PMID: 18367518 Free PMC article.
-
Activation of nuclear factor-kappaB p50 homodimer/Bcl-3 complexes in nasopharyngeal carcinoma.Cancer Res. 2003 Dec 1;63(23):8293-301. Cancer Res. 2003. PMID: 14678988
-
LMP1 TRAFficking activates growth and survival pathways.Adv Exp Med Biol. 2007;597:173-87. doi: 10.1007/978-0-387-70630-6_14. Adv Exp Med Biol. 2007. PMID: 17633026 Review.
-
The Latent Membrane Protein 1 (LMP1).Curr Top Microbiol Immunol. 2015;391:119-49. doi: 10.1007/978-3-319-22834-1_4. Curr Top Microbiol Immunol. 2015. PMID: 26428373 Review.
Cited by
-
Changes in expression induced by Epstein-Barr Virus LMP1-CTAR1: potential role of bcl3.mBio. 2015 Apr 14;6(2):e00441-15. doi: 10.1128/mBio.00441-15. mBio. 2015. PMID: 25873381 Free PMC article.
-
Epstein-Barr Virus Mediated Signaling in Nasopharyngeal Carcinoma Carcinogenesis.Cancers (Basel). 2020 Aug 28;12(9):2441. doi: 10.3390/cancers12092441. Cancers (Basel). 2020. PMID: 32872147 Free PMC article. Review.
-
Nuclear factor-kappaB binds to the Epstein-Barr Virus LMP1 promoter and upregulates its expression.J Virol. 2009 Feb;83(3):1393-401. doi: 10.1128/JVI.01637-08. Epub 2008 Nov 19. J Virol. 2009. PMID: 19019967 Free PMC article.
-
Quantitative study of cytotoxic T-lymphocyte immunotherapy for nasopharyngeal carcinoma.Theor Biol Med Model. 2012 Mar 7;9:6. doi: 10.1186/1742-4682-9-6. Theor Biol Med Model. 2012. PMID: 22394427 Free PMC article.
-
Epstein-Barr virus latent membrane protein 1 modulates distinctive NF- kappaB pathways through C-terminus-activating region 1 to regulate epidermal growth factor receptor expression.J Virol. 2010 Jul;84(13):6605-14. doi: 10.1128/JVI.00344-10. Epub 2010 Apr 21. J Virol. 2010. PMID: 20410275 Free PMC article.
References
-
- Atkinson, P. G., H. J. Coope, M. Rowe, and S. C. Ley. 2003. Latent membrane protein 1 of Epstein-Barr virus stimulates processing of NF-kappa B2 p100 to p52. J. Biol. Chem. 278:51134-51142. - PubMed
-
- Bours, V., G. Franzoso, V. Azarenko, S. Park, T. Kanno, K. Brown, and U. Siebenlist. 1993. The oncoprotein Bcl-3 directly transactivates through kappa B motifs via association with DNA-binding p50B homodimers. Cell 72:729-739. - PubMed
-
- Cogswell, P. C., D. C. Guttridge, W. K. Funkhouser, and A. S. Baldwin, Jr. 2000. Selective activation of NF-kappa B subunits in human breast cancer: potential roles for NF-kappa B2/p52 and for Bcl-3. Oncogene 19:1123-1131. - PubMed
-
- Dawson, C. W., G. Tramountanis, A. G. Eliopoulos, and L. S. Young. 2003. Epstein-Barr virus latent membrane protein 1 (LMP1) activates the phosphatidylinositol 3-kinase/Akt pathway to promote cell survival and induce actin filament remodeling. J. Biol. Chem. 278:3694-3704. - PubMed
-
- Eliopoulos, A. G., J. H. Caamano, J. Flavell, G. M. Reynolds, P. G. Murray, J. L. Poyet, and L. S. Young. 2003. Epstein-Barr virus-encoded latent infection membrane protein 1 regulates the processing of p100 NF-kappaB2 to p52 via an IKKgamma/NEMO-independent signalling pathway. Oncogene 22:7557-7569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

