Use of antibody avidity assays for diagnosis of severe acute respiratory syndrome coronavirus infection
- PMID: 17881505
- PMCID: PMC2168165
- DOI: 10.1128/CVI.00056-07
Use of antibody avidity assays for diagnosis of severe acute respiratory syndrome coronavirus infection
Abstract
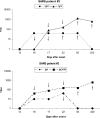
An indirect immunofluorescent assay (Euroimmun AG, Luebeck, Germany) was used to investigate the avidity of immunoglobulin G (IgG), IgM, IgA, and total Ig (IgGAM) antibody responses to severe acute respiratory syndrome coronavirus (SARS CoV) infections. Serial serum samples from eight patients collected during the first, third, and ninth months after the onset of infection were evaluated. It was found that low-avidity IgG antibodies were detected in 15/15 (100%), 1/5 (20%), and 0/8 (0%) serum samples collected during the first, third, and ninth months after the onset of symptoms, respectively. Low-avidity antibodies of IgA and IgM subclasses were detected in 14/14 (100%) and 3/14 (21%) serum samples, respectively, collected in the first month after the onset of infection. However, IgA antibodies remained low in avidity in a proportion of patients even during late convalescence. As a consequence, IgG antibody avidity assays gave better discrimination between acute-phase and late-convalescent-phase serum samples than IgM, IgA, or IgGAM assays. In two of these patients, sequential serum samples were also tested for IgG avidity against human CoV strains OC43 and 229E in parallel. While SARS CoV infections induced an anamnestic IgG antibody response to the 229E and OC43 viruses, these cross-reactive antibodies remained of high avidity from early (the first month) postinfection. The results showed that assays to detect low-avidity antibody may be useful for discriminating early from late antibody responses and also for distinguishing anamnestic cross-reactive antibody responses from primary specific responses. This may be useful in some clinical situations.
Figures
References
-
- Blackburn, N. K., T. G. Besselaar, B. D. Schoub, and K. F. O'Connell. 1991. Differentiation of primary cytomegalovirus infection from reactivation using the urea denaturation test for measuring antibody avidity. J. Med. Virol. 33:6-9. - PubMed
-
- Chan, K. H., V. C. C. Cheng, P. C. Y. Woo, S. K. P. Lau, L. L. M. Poon, Y. Guan, W. H. Seto, K. Y. Yuen, and J. S. M. Peiris. 2005. Serological responses in patients with severe acute respiratory syndrome coronavirus infection and cross-reactivity with human coronaviruses 229E, OC43, and NL63. Clin. Diagn. Lab. Immunol. 12:1317-1321. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

