Signaling properties of a short-wave cone visual pigment and its role in phototransduction
- PMID: 17881515
- PMCID: PMC6672674
- DOI: 10.1523/JNEUROSCI.2211-07.2007
Signaling properties of a short-wave cone visual pigment and its role in phototransduction
Abstract
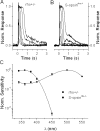
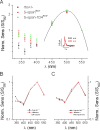
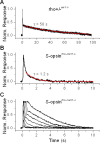
Although visual pigments play key structural and functional roles in photoreceptors, the relationship between the properties of mammalian cone pigments and those of mammalian cones is not well understood. We generated transgenic mice with rods expressing mouse short-wave cone opsin (S-opsin) to test whether cone pigment can substitute for the structural and functional roles of rhodopsin and to investigate how the biophysical and signaling properties of the short-wave cone pigment (S-pigment) contribute to the specialized function of cones. The transgenic S-opsin was targeted to rod outer segments, and formed a pigment with peak absorption at 360 nm. Expression of S-opsin in rods lacking rhodopsin (rho-/-) promoted outer segment growth and cell survival and restored their ability to respond to light while shifting their action spectrum to 355 nm. Using the spectral separation between S-pigment and rhodopsin, we found that the two pigments produced similar photoresponses. Dark noise did not increase in transgenic rods, indicating that thermal activation of S-pigment might not contribute to the low sensitivity of mouse S-cones. Using rod arrestin knock-out animals (arr1-/-), we found that the physiologically active (meta II) state of S-pigment decays 40 times faster than that of rhodopsin. Interestingly, rod arrestin was efficient in deactivating S-pigment in rods, but its deletion did not have any obvious effect on dim-flash response shutoff in cones. Furthermore, transgenic cone arrestin was not able to rescue the slow shutoff of S-pigment dim-flash response in arr1-/- rods. Thus, the connection between rod/cone arrestins and S-pigment shutoff remains unclear.
Figures




References
-
- Burns ME, Mendez A, Chen J, Baylor DA. Dynamics of cyclic GMP synthesis in retinal rods. Neuron. 2002;36:81–91. - PubMed
-
- Calvert PD, Govardovskii VI, Krasnoperova N, Anderson RE, Lem J, Makino CL. Membrane protein diffusion sets the speed of rod phototransduction. Nature. 2001;411:90–94. - PubMed
-
- Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J Comp Neurol. 1979;188:245–262. - PubMed
-
- Concepcion F, Mendez A, Chen J. The carboxyl-terminal domain is essential for rhodopsin transport in rod photoreceptors. Vision Res. 2002;42:417–426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
