System x(c)- activity and astrocytes are necessary for interleukin-1 beta-mediated hypoxic neuronal injury
- PMID: 17881516
- PMCID: PMC6672668
- DOI: 10.1523/JNEUROSCI.2459-07.2007
System x(c)- activity and astrocytes are necessary for interleukin-1 beta-mediated hypoxic neuronal injury
Abstract
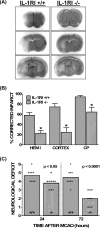
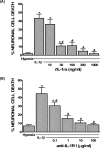
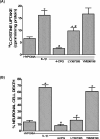
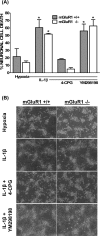
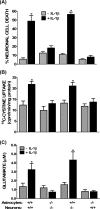
The purpose of this study was to elucidate the cellular/biochemical pathway(s) by which interleukin-1beta (IL-1beta) contributes to the pathogenesis of hypoxic-ischemic brain damage. In vivo, IL-1 receptor type I (IL-1RI)-deficient mice showed smaller infarcts and less neurological deficits than wild-type animals after a 90 min reversible middle cerebral artery occlusion. In vitro, IL-1beta mediated an enhancement of hypoxic neuronal injury in murine cortical cultures that was lacking in cultures derived from IL-1RI null mutant animals and was blocked by the IL-1 receptor antagonist or an IL-1RI blocking antibody. This IL-1beta-mediated potentiation of hypoxic neuronal injury was associated with an increase in both cellular cystine uptake ([cystine]i) and extracellular glutamate levels ([glutamate]e) and was prevented by either ionotropic glutamate receptor antagonism or removal of L-cystine, suggesting a role for the cystine/glutamate antiporter (System x(c)-). Indeed, dual System x(c)-/metabotropic glutamate receptor subunit 1 (mGluR1) antagonism but not selective mGluR1 antagonism prevented neuronal injury. Additionally, cultures derived from mGluR1-deficient mice exhibited the same potentiation in injury after treatment with IL-1beta as wild-type cultures, an effect prevented by System x(c)-/mGluR1 antagonism. Finally, assessment of System x(c)- function and kinetics in IL-1beta-treated cultures revealed an increase in velocity of cystine transport (Vmax), in the absence of a change in affinity (Km). Neither the enhancement in [cystine]i, [glutamate]e, or neuronal injury were observed in chimeric cultures consisting of IL-1RI(+/+) neurons plated on top of IL-1RI(-/-) astrocytes, highlighting the importance of astrocyte-mediated alterations in System x(c)- as a novel contributor to the development and progression of hypoxic neuronal injury.
Figures




References
-
- Andre R, Moggs JG, Kimber I, Rothwell NJ, Pinteaux E. Gene regulation by IL-1beta independent of IL-1R1 in the mouse brain. Glia. 2006;53:477–483. - PubMed
-
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. - PubMed
-
- Arend WP. Interleukin-1 receptor antagonist. Adv Immunol. 1993;54:167–227. - PubMed
-
- Baird AE, Benfield A, Schlaug G, Siewert B, Lovblad KO, Edelman RR, Warach S. Enlargement of human cerebral ischemic lesion volumes measured by diffusion-weighted magnetic resonance imaging. Ann Neurol. 1997;41:581–589. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
