A biologically realistic model of contrast invariant orientation tuning by thalamocortical synaptic depression
- PMID: 17881529
- PMCID: PMC6672681
- DOI: 10.1523/JNEUROSCI.1640-07.2007
A biologically realistic model of contrast invariant orientation tuning by thalamocortical synaptic depression
Abstract
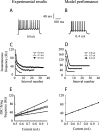
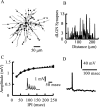
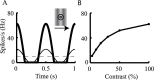
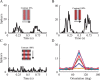
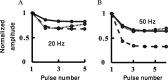
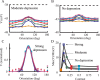
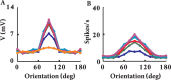
Simple cells in layer 4 of the primary visual cortex of the cat show contrast-invariant orientation tuning, in which the amplitude of the peak response is proportional to the stimulus contrast but the width of the tuning curve hardly changes with contrast. This study uses a detailed model of spiny stellate cells (SSCs) from cat area 17 to explain this property. The model integrates our experimental data, including morphological and intrinsic membrane properties and the number and spatial distribution of four major synaptic input sources of the SSC: the dorsal lateral geniculate nucleus (dLGN) and three cortical sources. The model also includes synaptic properties of these inputs. The cortical input served as sources of background activity, and visual stimuli was modeled as sinusoidal grating. For all contrasts, strong synaptic depression of the dLGN feedforward afferents compresses the firing rates in response to orthogonal stimuli, keeping these rates at practically the same low level. However, at preferred orientations, despite synaptic depression, firing rate changes as a function of contrast. Thus, when embedded in an active network, strong synaptic depression can explain contrast-invariant orientation tuning of simple cells. This is true also when the dLGN inputs are partially depressed as a result of their spontaneous activity and to some extent also when parameters were fitted to a more moderate level of synaptic depression. The model response is in close agreement with experimental results, in terms of both output spikes and membrane voltage (amplitude and fluctuations), with reasonable exceptions given that recurrent connections were not incorporated.
Figures



References
-
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. - PubMed
-
- Ahmed B, Anderson JC, Douglas RJ, Martin KA, Nelson JC. Polyneuronal innervation of spiny stellate neurons in cat visual cortex. J Comp Neurol. 1994;341:39–49. - PubMed
-
- Ahmed B, Anderson JC, Martin KA, Nelson JC. Map of the synapses onto layer 4 basket cells of the primary visual cortex of the cat. J Comp Neurol. 1997;380:230–242. - PubMed
-
- Ahmed B, Anderson JC, Douglas RJ, Martin KA, Whitteridge D. Estimates of the net excitatory currents evoked by visual stimulation of identified neurons in cat visual cortex. Cereb Cortex. 1998;8:462–476. - PubMed
-
- Anderson JC, Douglas RJ, Martin KA, Nelson JC. Map of the synapses formed with the dendrites of spiny stellate neurons of cat visual cortex. J Comp Neurol. 1994a;341:25–38. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
