Properties of GluR3 receptors tagged with GFP at the amino or carboxyl terminus
- PMID: 17881566
- PMCID: PMC2000508
- DOI: 10.1073/pnas.0706773104
Properties of GluR3 receptors tagged with GFP at the amino or carboxyl terminus
Abstract
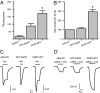
Anatomical visualization of neurotransmitter receptor localization is facilitated by tagging receptors, but this process can alter their functional properties. We have evaluated the distribution and properties of WT glutamate receptor 3 (GluR3) alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors (WT GluR3) and two receptors in which GFP was tagged to the amino terminus (GFP-GluR3) or to the carboxyl terminus (GluR3-GFP). Although the fluorescence in Xenopus oocytes was stronger in the vegetal hemisphere because of localization of internal structures (probable sites of production, storage or recycling of receptors), the insertion of receptors into the plasma membrane was polarized to the animal hemisphere. The fluorescence intensity of oocytes injected with GluR3-GFP RNA was approximately double that of oocytes injected with GFP-GluR3 RNA. Accordingly, GluR3-GFP oocytes generated larger kainate-induced currents than GFP-GluR3 oocytes, with similar EC(50) values. Currents elicited by glutamate, or AMPA coapplied with cyclothiazide, were also larger in GluR3-GFP oocytes. The glutamate- to kainate-current amplitude ratios differed, with GluR3-GFP being activated more efficiently by glutamate than the WT or GFP-GluR3 receptors. This pattern correlates with the slower decay of glutamate-induced currents generated by GluR3-GFP receptors. These changes were not observed when GFP was tagged to the amino terminus, and these receptors behaved like the WT. The antagonistic effects of 6-nitro-7-sulfamoylbenzo[f]quinoxaline-2,3-dione (NBQX) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) were not altered in any of the tagged receptors. We conclude that GFP is a useful and convenient tag for visualizing these proteins. However, the effects of different sites of tag insertion on receptor characteristics must be taken into account in assessing the roles played by these receptor proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


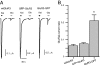
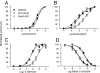
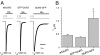
Similar articles
-
Complex pharmacological properties of recombinant alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor subtypes.Mol Pharmacol. 1992 Nov;42(5):864-71. Mol Pharmacol. 1992. PMID: 1279377
-
Glutamate-induced cobalt uptake elicited by kainate receptors in rat taste bud cells.Chem Senses. 2005 Feb;30(2):137-43. doi: 10.1093/chemse/bji009. Chem Senses. 2005. PMID: 15703333
-
The fast kinetics of AMPA GluR3 receptors is selectively modulated by the TARPs gamma 4 and gamma 8.Mol Cell Neurosci. 2008 May;38(1):117-23. doi: 10.1016/j.mcn.2008.01.018. Epub 2008 Mar 5. Mol Cell Neurosci. 2008. PMID: 18395463
-
Gamma-D-glutamylaminomethyl sulfonic acid (GAMS) distinguishes kainic acid- from AMPA-induced responses in Xenopus oocytes expressing chick brain glutamate receptors.Neuropharmacology. 1993 Aug;32(8):767-75. doi: 10.1016/0028-3908(93)90185-6. Neuropharmacology. 1993. PMID: 7692340
-
Competitive AMPA receptor antagonists.Med Res Rev. 2007 Mar;27(2):239-78. doi: 10.1002/med.20084. Med Res Rev. 2007. PMID: 16892196 Review.
Cited by
-
Chemical labelling for visualizing native AMPA receptors in live neurons.Nat Commun. 2017 Apr 7;8:14850. doi: 10.1038/ncomms14850. Nat Commun. 2017. PMID: 28387242 Free PMC article.
-
Impact of cell type and epitope tagging on heterologous expression of G protein-coupled receptor: a systematic study on angiotensin type II receptor.PLoS One. 2012;7(10):e47016. doi: 10.1371/journal.pone.0047016. Epub 2012 Oct 8. PLoS One. 2012. PMID: 23056563 Free PMC article.
-
Distinct subcellular distribution of delta-opioid receptor fused with various tags in PC12 cells.Neurochem Res. 2008 Oct;33(10):2028-34. doi: 10.1007/s11064-008-9678-9. Epub 2008 Mar 26. Neurochem Res. 2008. PMID: 18365312
-
Using fluorometry and ion-sensitive microelectrodes to study the functional expression of heterologously-expressed ion channels and transporters in Xenopus oocytes.Methods. 2010 May;51(1):134-45. doi: 10.1016/j.ymeth.2009.12.012. Epub 2010 Jan 4. Methods. 2010. PMID: 20051266 Free PMC article. Review.
-
Electrophysiological evaluation of extracellular spermine and alkaline pH on synaptic human GABAA receptors.Transl Psychiatry. 2019 Sep 5;9(1):218. doi: 10.1038/s41398-019-0551-1. Transl Psychiatry. 2019. PMID: 31488811 Free PMC article.
References
-
- Ferraguti F, Shigemoto R. Cell Tissue Res. 2006;326:483–504. - PubMed
-
- Dingledine R, Borges K, Bowie D, Traynelis SF. Pharmacol Rev. 1999;51:7–61. - PubMed
-
- Jonas P, Burnashev N. Neuron. 1995;15:987–990. - PubMed
-
- Bennett JA, Dingledine R. Neuron. 1995;14:373–384. - PubMed
-
- Cull-Candy S, Kelly L, Farrant M. Curr Opin Neurobiol. 2006;16:288–297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

