Myosin VI is required for targeted membrane transport during cytokinesis
- PMID: 17881731
- PMCID: PMC2096599
- DOI: 10.1091/mbc.e07-02-0127
Myosin VI is required for targeted membrane transport during cytokinesis
Abstract
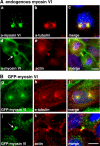
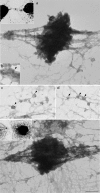
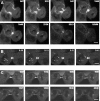
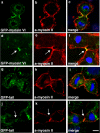
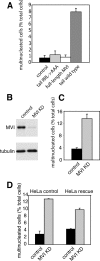
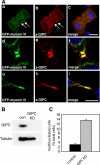
Myosin VI plays important roles in endocytic and exocytic membrane-trafficking pathways in cells. Because recent work has highlighted the importance of targeted membrane transport during cytokinesis, we investigated whether myosin VI plays a role in this process during cell division. In dividing cells, myosin VI undergoes dramatic changes in localization: in prophase, myosin VI is recruited to the spindle poles; and in cytokinesis, myosin VI is targeted to the walls of the ingressing cleavage furrow, with a dramatic concentration in the midbody region. Furthermore, myosin VI is present on vesicles moving into and out of the cytoplasmic bridge connecting the two daughter cells. Inhibition of myosin VI activity by small interfering RNA (siRNA)-mediated knockdown or by overexpression of dominant-negative myosin VI tail leads to a delay in metaphase progression and a defect in cytokinesis. GAIP-interacting protein COOH terminus (GIPC), a myosin VI binding partner, is associated with the function(s) of myosin VI in dividing cells. Loss of GIPC in siRNA knockdown cells results in a more than fourfold increase in the number of multinucleated cells. Our results suggest that myosin VI has novel functions in mitosis and that it plays an essential role in targeted membrane transport during cytokinesis.
Figures


References
-
- Albertson R., Riggs B., Sullivan W. Membrane traffic: a driving force in cytokinesis. Trends Cell Biol. 2005;15:92–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

