Late endosomal traffic of the epidermal growth factor receptor ensures spatial and temporal fidelity of mitogen-activated protein kinase signaling
- PMID: 17881733
- PMCID: PMC2096590
- DOI: 10.1091/mbc.e07-02-0098
Late endosomal traffic of the epidermal growth factor receptor ensures spatial and temporal fidelity of mitogen-activated protein kinase signaling
Abstract
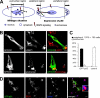
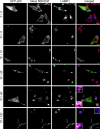
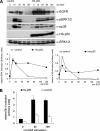
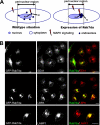
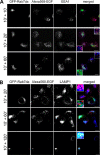
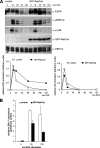
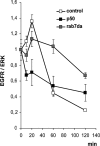
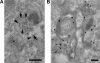
Mitogen-activated protein kinase (MAPK) signaling is regulated by assembling distinct scaffold complexes at the plasma membrane and on endosomes. Thus, spatial resolution might be critical to determine signaling specificity. Therefore, we investigated whether epidermal growth factor receptor (EGFR) traffic through the endosomal system provides spatial information for MAPK signaling. To mislocalize late endosomes to the cell periphery we used the dynein subunit p50 dynamitin. The peripheral translocation of late endosomes resulted in a prolonged EGFR activation on late endosomes and a slow down in EGFR degradation. Continuous EGFR signaling from late endosomes caused sustained extracellular signal-regulated kinase and p38 signaling and resulted in hyperactivation of nuclear targets, such as Elk-1. In contrast, clustering late endosomes in the perinuclear region by expression of dominant active Rab7 delayed the entry of the EGFR into late endosomes, which caused a delay in EGFR degradation and a sustained MAPK signaling. Surprisingly, the activation of nuclear targets was reduced. Thus, we conclude that appropriate trafficking of the activated EGFR through endosomes controls the spatial and temporal regulation of MAPK signaling.
Figures


References
-
- Adachi T., Kar S., Wang M., Carr B. I. Transient and sustained ERK phosphorylation and nuclear translocation in growth control. J. Cell Physiol. 2002;192:151–159. - PubMed
-
- Bohn G., et al. A novel human primary immunodeficiency syndrome caused by deficiency of the endosomal adaptor protein p14. Nat. Med. 2007;13:38–45. - PubMed
-
- Buchwalter G., Gross C., Wasylyk B. Ets ternary complex transcription factors. Gene. 2004;324:1–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

