Generation of functional multipotent adult stem cells from GPR125+ germline progenitors
- PMID: 17882221
- PMCID: PMC2935199
- DOI: 10.1038/nature06129
Generation of functional multipotent adult stem cells from GPR125+ germline progenitors
Abstract
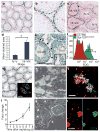
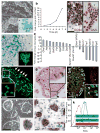
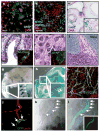
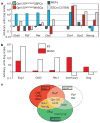
Adult mammalian testis is a source of pluripotent stem cells. However, the lack of specific surface markers has hampered identification and tracking of the unrecognized subset of germ cells that gives rise to multipotent cells. Although embryonic-like cells can be derived from adult testis cultures after only several weeks in vitro, it is not known whether adult self-renewing spermatogonia in long-term culture can generate such stem cells as well. Here, we show that highly proliferative adult spermatogonial progenitor cells (SPCs) can be efficiently obtained by cultivation on mitotically inactivated testicular feeders containing CD34+ stromal cells. SPCs exhibit testicular repopulating activity in vivo and maintain the ability in long-term culture to give rise to multipotent adult spermatogonial-derived stem cells (MASCs). Furthermore, both SPCs and MASCs express GPR125, an orphan adhesion-type G-protein-coupled receptor. In knock-in mice bearing a GPR125-beta-galactosidase (LacZ) fusion protein under control of the native Gpr125 promoter (GPR125-LacZ), expression in the testis was detected exclusively in spermatogonia and not in differentiated germ cells. Primary GPR125-LacZ SPC lines retained GPR125 expression, underwent clonal expansion, maintained the phenotype of germline stem cells, and reconstituted spermatogenesis in busulphan-treated mice. Long-term cultures of GPR125+ SPCs (GSPCs) also converted into GPR125+ MASC colonies. GPR125+ MASCs generated derivatives of the three germ layers and contributed to chimaeric embryos, with concomitant downregulation of GPR125 during differentiation into GPR125- cells. MASCs also differentiated into contractile cardiac tissue in vitro and formed functional blood vessels in vivo. Molecular bookmarking by GPR125 in the adult mouse and, ultimately, in the human testis could enrich for a population of SPCs for derivation of GPR125+ MASCs, which may be employed for genetic manipulation, tissue regeneration and revascularization of ischaemic organs.
Figures
References
-
- Guan K, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–1203. - PubMed
-
- Kanatsu-Shinohara M, Shinohara T. The germ of pluripotency. Nature Biotechnol. 2006;24:663–664. - PubMed
-
- Valenzuela DM, et al. High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nature Biotechnol. 2003;21:652–659. - PubMed
-
- Oakberg EF. A description of spermiogenesis in the mouse and its use in analysis of the cycle of the seminiferous epithelium and germ cell renewal. Am J Anat. 1956;99:391–413. - PubMed
-
- Fiering SN, et al. Improved FACS-Gal: flow cytometric analysis and sorting of viable eukaryotic cells expressing reporter gene constructs. Cytometry. 1991;12:291–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

