Glycosyltransferase mechanisms: impact of a 5-fluoro substituent in acceptor and donor substrates on catalysis
- PMID: 17883281
- PMCID: PMC2556460
- DOI: 10.1021/bi700863s
Glycosyltransferase mechanisms: impact of a 5-fluoro substituent in acceptor and donor substrates on catalysis
Abstract
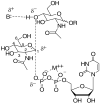
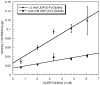
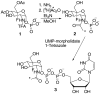
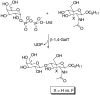
In glycosyltransferase-catalyzed reactions a new carbohydrate-carbohydrate bond is formed between a carbohydrate acceptor and the carbohydrate moiety of either a sugar nucleotide donor or lipid-linked saccharide donor. It is currently believed that most glycosyltransferase-catalyzed reactions occur via an electrophilic activation mechanism with the formation of an oxocarbenium ion-like transition state, a hypothesis that makes clear predictions regarding the charge development on the donor (strong positive charge) and acceptor (minimal negative charge) substrates. To better understand the mechanism of these enzyme-catalyzed reactions, we have introduced a strongly electron-withdrawing group (fluorine) at C-5 of both donor and acceptor substrates in order to explore its effect on catalysis. In particular, we have investigated the effects of the 5-fluoro analogues on the kinetics of two glycosyltransferase-catalyzed reactions mediated by UDP-GlcNAc:GlcNAc-P-P-Dol N-acetylglucosaminyltransferase (chitobiosyl-P-P-lipid synthase, CLS) and beta-N-acetylglucosaminyl-beta-1,4 galactosyltransferase (GalT). The 5-fluoro group has a marked effect on catalysis when inserted into the UDP-GlcNAc donor, with the UDP(5-F)-GlcNAc serving as a competitive inhibitor of CLS rather than a substrate. The (5-F)-GlcNAc beta-octyl glycoside acceptor, however, is an excellent substrate for GalT. Both of these results support a weakly associative transition state for glycosyltransferase-catalyzed reactions that proceed with inversion of configuration.
Figures

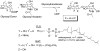
References
-
- Slawson C, Housley MP, Hart GW. O-GlcNAc cycling: how a single sugar post-translational modification is changing the way we think about signalling networks. J Cell Biochem. 2006;97:71–83. - PubMed
-
- Hancock IC. Bacterial cell surface carbohydrates: structure and assembly. Biochem Soc Trans. 1997;25 - PubMed
-
- Vogt T, Jones P. Glycosyltransferases in plant natural product synthesis: characterization of a supergene family. Trends Plant Sci. 2000;5:380–386. - PubMed
-
- Coutinho PM, Deleury E, Davies GJ, Henrissat B. An evolving hierarchical family classification for glycosyltransferases. J Mol Biol. 2003;328:307–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

