Faced with inequality: chicken do not have a general dosage compensation of sex-linked genes
- PMID: 17883843
- PMCID: PMC2099419
- DOI: 10.1186/1741-7007-5-40
Faced with inequality: chicken do not have a general dosage compensation of sex-linked genes
Abstract
Background: The contrasting dose of sex chromosomes in males and females potentially introduces a large-scale imbalance in levels of gene expression between sexes, and between sex chromosomes and autosomes. In many organisms, dosage compensation has thus evolved to equalize sex-linked gene expression in males and females. In mammals this is achieved by X chromosome inactivation and in flies and worms by up- or down-regulation of X-linked expression, respectively. While otherwise widespread in systems with heteromorphic sex chromosomes, the case of dosage compensation in birds (males ZZ, females ZW) remains an unsolved enigma.
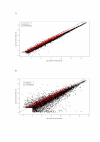
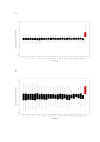
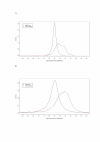
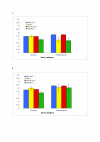
Results: Here, we use a microarray approach to show that male chicken embryos generally express higher levels of Z-linked genes than female birds, both in soma and in gonads. The distribution of male-to-female fold-change values for Z chromosome genes is wide and has a mean of 1.4-1.6, which is consistent with absence of dosage compensation and sex-specific feedback regulation of gene expression at individual loci. Intriguingly, without global dosage compensation, the female chicken has significantly lower expression levels of Z-linked compared to autosomal genes, which is not the case in male birds.
Conclusion: The pronounced sex difference in gene expression is likely to contribute to sexual dimorphism among birds, and potentially has implication to avian sex determination. Importantly, this report, together with a recent study of sex-biased expression in somatic tissue of chicken, demonstrates the first example of an organism with a lack of global dosage compensation, providing an unexpected case of a viable system with large-scale imbalance in gene expression between sexes.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

