Effects of mu- and kappa-2 opioid receptor agonists on pain and rearing behaviors
- PMID: 17883847
- PMCID: PMC2045099
- DOI: 10.1186/1744-9081-3-49
Effects of mu- and kappa-2 opioid receptor agonists on pain and rearing behaviors
Abstract
Background: Management of pain involves a balance between inhibition of pain and minimization of side effects; therefore, in developing new analgesic compounds, one must consider the effects of treatment on both pain processing and behavior. The purpose of this study was to evaluate the effects of the mu and kappa-2 opioid receptor agonists on general and pain behavioral outcomes.
Methods: As a general behavioral assessment, we modified the cylinder rearing assay and recorded the number and duration of rearing events. Thermal sensitivity was evaluated using either a reflexive measure of hindpaw withdrawal latency to a radiant heat source or using an orofacial operant thermal assay. Acetic acid-induced visceral pain and capsaicin-induced neurogenic inflammatory pain were used as painful stimuli. The mu-opioid receptor agonist, morphine or the kappa-2 receptor agonist GR89696 was administered 30 min prior to testing. A general linear model repeated measures analysis was completed for baseline session comparisons and an analysis of variance was used to evaluate the effects of treatment on each outcome measure (SPSS Inc). When significant differences were found, post-hoc comparisons were made using the Tukey honestly significant difference test. *P < 0.05 was considered significant in all instances.
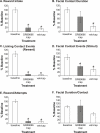
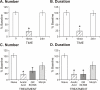
Results: We found that morphine and GR89,696 dose-dependently decreased the number of reaching events and rearing duration. Rearing behavior was not affected at 0.5 mg/kg for morphine, 1.25 x 10-4 mg/kg for GR89,696. Hindpaw thermal sensitivity was significantly increased only at the highest doses for each drug. At the highest dose that did not significantly influence rearing behavior, we found that visceral and neurogenic inflammatory pain was not affected following GR89,696 administration and morphine was only partially effective for blocking visceral pain.
Conclusion: This study demonstrated that high levels of the opioids produced significant untoward effects and made distinguishing an analgesic versus a more general effect more difficult. Quantification of rearing behavior in conjunction with standard analgesic assays can help in gaining a better appreciation of true analgesic efficacy of experimental drugs.
Figures



Similar articles
-
Antinociceptive and adverse effects of mu- and kappa-opioid receptor agonists: a comparison of morphine and U50488-H.Basic Clin Pharmacol Toxicol. 2008 Nov;103(5):419-27. doi: 10.1111/j.1742-7843.2008.00306.x. Epub 2008 Aug 11. Basic Clin Pharmacol Toxicol. 2008. PMID: 18699797
-
Morphine can produce analgesia via spinal kappa opioid receptors in the absence of mu opioid receptors.Brain Res. 2006 Apr 14;1083(1):61-9. doi: 10.1016/j.brainres.2006.01.095. Epub 2006 Mar 10. Brain Res. 2006. PMID: 16530171
-
Low-Dose Cannabinoid Type 2 Receptor Agonist Attenuates Tolerance to Repeated Morphine Administration via Regulating μ-Opioid Receptor Expression in Walker 256 Tumor-Bearing Rats.Anesth Analg. 2016 Apr;122(4):1031-7. doi: 10.1213/ANE.0000000000001129. Anesth Analg. 2016. PMID: 26720619
-
Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone).Pain Pract. 2008 Jul-Aug;8(4):287-313. doi: 10.1111/j.1533-2500.2008.00204.x. Epub 2008 May 23. Pain Pract. 2008. PMID: 18503626
-
"Weak" opioid analgesics. Codeine, dihydrocodeine and tramadol: no less risky than morphine.Prescrire Int. 2016 Feb;25(168):45-50. Prescrire Int. 2016. PMID: 27042732 Review.
Cited by
-
Antinociceptive Effects of Kappa-Opioid Receptor Agonists.Handb Exp Pharmacol. 2022;271:293-313. doi: 10.1007/164_2020_430. Handb Exp Pharmacol. 2022. PMID: 33387069 Review.
-
Pharmacological Characterization of Orofacial Nociception in Female Rats Following Nitroglycerin Administration.Front Pharmacol. 2020 Dec 3;11:527495. doi: 10.3389/fphar.2020.527495. eCollection 2020. Front Pharmacol. 2020. PMID: 33343340 Free PMC article.
-
Influence of social interaction on nociceptive-induced changes in locomotor activity in a mouse model of acute inflammatory pain: Use of novel thermal assays.Brain Res Bull. 2017 Sep;134:47-54. doi: 10.1016/j.brainresbull.2017.06.017. Epub 2017 Jun 23. Brain Res Bull. 2017. PMID: 28652168 Free PMC article.
-
Phosphorylation of the N-methyl-d-aspartate receptor is increased in the nucleus accumbens during both acute and extended morphine withdrawal.J Pharmacol Exp Ther. 2015 Dec;355(3):496-505. doi: 10.1124/jpet.115.227629. Epub 2015 Sep 16. J Pharmacol Exp Ther. 2015. PMID: 26377910 Free PMC article.
-
Central sensitization in the trigeminal nucleus caudalis produced by a conjugate of substance P and the A subunit of cholera toxin.J Pain. 2010 Sep;11(9):838-46. doi: 10.1016/j.jpain.2010.05.007. J Pain. 2010. PMID: 20620120 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

