Multicopy suppression underpins metabolic evolvability
- PMID: 17884825
- PMCID: PMC2678898
- DOI: 10.1093/molbev/msm204
Multicopy suppression underpins metabolic evolvability
Abstract
Our understanding of the origins of new metabolic functions is based upon anecdotal genetic and biochemical evidence. Some auxotrophies can be suppressed by overexpressing substrate-ambiguous enzymes (i.e., those that catalyze the same chemical transformation on different substrates). Other enzymes exhibit weak but detectable catalytic promiscuity in vitro (i.e., they catalyze different transformations on similar substrates). Cells adapt to novel environments through the evolution of these secondary activities, but neither their chemical natures nor their frequencies of occurrence have been characterized en bloc. Here, we systematically identified multifunctional genes within the Escherichia coli genome. We screened 104 single-gene knockout strains and discovered that many (20%) of these auxotrophs were rescued by the overexpression of at least one noncognate E. coli gene. The deleted gene and its suppressor were generally unrelated, suggesting that promiscuity is a product of contingency. This genome-wide survey demonstrates that multifunctional genes are common and illustrates the mechanistic diversity by which their products enhance metabolic robustness and evolvability.
Figures
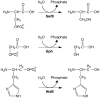
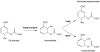
References
-
- Aronson BD, Somerville RL, Epperly BR, Dekker EE. The primary structure of Escherichia colil-threonine dehydrogenase. J Biol Chem. 1989;264:5226–5232. - PubMed
-
- Berg CM, Wang MD, Vartak NB, Liu L. Acquisition of new metabolic capabilities: multicopy suppression by cloned transaminase genes in Escherichia coli K-12. Gene. 1988;65:195–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

