Chromophore switch from 11-cis-dehydroretinal (A2) to 11-cis-retinal (A1) decreases dark noise in salamander red rods
- PMID: 17884920
- PMCID: PMC2375465
- DOI: 10.1113/jphysiol.2007.142935
Chromophore switch from 11-cis-dehydroretinal (A2) to 11-cis-retinal (A1) decreases dark noise in salamander red rods
Abstract
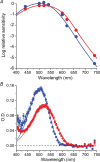
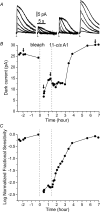
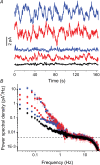
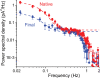
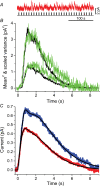
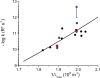
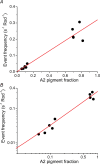
Dark noise, light-induced noise and responses to brief flashes of light were recorded in the membrane current of isolated rods from larval tiger salamander retina before and after bleaching most of the native visual pigment, which mainly has the 11-cis-3,4-dehydroretinal (A2) chromophore, and regenerating with the 11-cis-retinal (A1) chromophore in the same isolated rods. The purpose was to test the hypothesis that blue-shifting the pigment by switching from A2 to A1 will decrease the rate of spontaneous thermal activations and thus intrinsic light-like noise in the rod. Complete recordings were obtained in five cells (21 degrees C). Based on the wavelength of maximum absorbance, lambda max,A1 = 502 nm and lambda max,A2 = 528 nm, the average A2 : A1 ratio determined from rod spectral sensitivities and absorbances was approximately 0.74 : 0.26 in the native state and approximately 0.09 : 0.91 in the final state. In the native (A2) state, the single-quantum response (SQR) had an amplitude of 0.41 +/- 0.03 pA and an integration time of 3.16 +/- 0.15 s (mean +/- s.e.m.). The low-frequency branch of the dark noise power spectrum was consistent with discrete SQR-like events occurring at a rate of 0.238 +/- 0.026 rod(-1) s(-1). The corresponding values in the final state were 0.57 +/- 0.07 pA (SQR amplitude), 3.47 +/- 0.26 s (SQR integration time), and 0.030 +/- 0.006 rod(-1) s(-1) (rate of dark events). Thus the rate of dark events per rod and the fraction of A2 pigment both changed by ca 8-fold between the native and final states, indicating that the dark events originated mainly in A2 molecules even in the final state. By extrapolating the linear relation between event rates and A2 fraction to 0% A2 (100% A1) and 100% A2 (0% A1), we estimated that the A1 pigment is at least 36 times more stable than the A2 pigment. The noise component attributed to discrete dark events accounted for 73% of the total dark current variance in the native (A2) state and 46% in the final state. The power spectrum of the remaining 'continuous' noise component did not differ between the two states. The smaller and faster SQR in the native (A2) state is consistent with the idea that the rod behaves as if light-adapted by dark events that occur at a rate of nearly one per integration time. Both the decreased level of dark noise and the increased SQR amplitude must significantly improve the reliability of photon detection in dim light in the presence of the A1 chromophore compared to the native (A2) state in salamander rods.
Figures
References
-
- Aho AC, Donner K, Helenius S, Olesen Larsen L, Reuter T. Visual performance of the toad (Bufo bufo) at low light levels: retinal ganglion cell responses and prey-catching accuracy. J Comp Physiol [A] 1993;172:671–682. - PubMed
-
- Aho AC, Donner K, Hyden C, Larsen LO, Reuter T. Low retinal noise in animals with low body temperature allows high visual sensitivity. Nature. 1988;334:348–350. - PubMed
-
- Ala-Laurila P, Pahlberg J, Koskelainen A, Donner K. On the relation between the photoactivation energy and the absorbance spectrum of visual pigments. Vision Res. 2004b;44:2153–2158. - PubMed
-
- Barlow HB. Retinal noise and absolute threshold. J Opt Soc Am. 1956;46:634–639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

