Expression and biological activity of ABCA1 in alveolar epithelial cells
- PMID: 17884990
- PMCID: PMC2258448
- DOI: 10.1165/rcmb.2007-0020OC
Expression and biological activity of ABCA1 in alveolar epithelial cells
Abstract
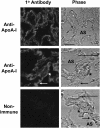
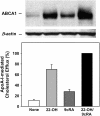
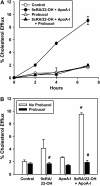
The mechanisms used by alveolar type I pneumocytes for maintenance of the lipid homeostasis necessary to sustain these large squamous cells are unknown. The processes may involve the ATP-binding cassette transporter A1 (ABCA1), a transport protein shown to be crucial in apolipoprotein A-I (apoA-I)-mediated mobilization of cellular cholesterol and phospholipid. Immunohistochemical data demonstrated the presence of ABCA1 in lung type I and type II cells and in cultured pneumocytes. Type II cells isolated from rat lungs and cultured for 5 days in 10% serum trans-differentiated toward cells with a type I-like phenotype which reacted with the type I cell-specific monoclonal antibody VIIIB2. Upon incubation of the type I-like pneumocytes with agents that up-regulate the ABCA1 gene (9-cis-retinoic acid [9cRA] and 22-hydroxycholesterol [22-OH, 9cRA/22-OH]), ABCA1 protein levels were enhanced to maximum levels after 8 to 16 hours and remained elevated for 24 hours. In the presence of apoA-I and 9cRA/22-OH, efflux of radioactive phospholipid and cholesterol from pneumocytes was stimulated 3- to 20-fold, respectively, over controls. Lipid efflux was inhibited by Probucol. Sucrose density gradient analysis of the media from stimulated cells incubated with apoA-I identified heterogeneous lipid particles that isolated at a density between 1.063 and 1.210 g/ml, with low or high apoA-I content. Thus, pneumocytes with markers for the type I phenotype contained functional ABCA1 protein, released lipid to apoA-I protein, and were capable of producing particles resembling nascent high-density lipoprotein, indicating an important role for ABCA1 in the maintenance of lung lipid homeostasis.
Figures





Similar articles
-
Identification and characterization of rodent ABCA1 in isolated type II pneumocytes.Am J Physiol Lung Cell Mol Physiol. 2003 Oct;285(4):L869-78. doi: 10.1152/ajplung.00077.2003. Epub 2003 Aug 8. Am J Physiol Lung Cell Mol Physiol. 2003. PMID: 12909583
-
Molecular and cellular physiology of apolipoprotein A-I lipidation by the ATP-binding cassette transporter A1 (ABCA1).J Biol Chem. 2004 Feb 27;279(9):7384-94. doi: 10.1074/jbc.M306963200. Epub 2003 Dec 4. J Biol Chem. 2004. PMID: 14660648
-
22R-hydroxycholesterol and 9-cis-retinoic acid induce ATP-binding cassette transporter A1 expression and cholesterol efflux in brain cells and decrease amyloid beta secretion.J Biol Chem. 2003 Apr 11;278(15):13244-56. doi: 10.1074/jbc.M300044200. Epub 2003 Jan 22. J Biol Chem. 2003. PMID: 12547833
-
Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL.J Mol Med (Berl). 2006 Apr;84(4):276-94. doi: 10.1007/s00109-005-0030-4. Epub 2006 Feb 25. J Mol Med (Berl). 2006. PMID: 16501936 Review.
-
Cellular cholesterol substrate pools for adenosine-triphosphate cassette transporter A1-dependent high-density lipoprotein formation.Curr Opin Lipidol. 2008 Jun;19(3):270-6. doi: 10.1097/MOL.0b013e3282feea99. Curr Opin Lipidol. 2008. PMID: 18460918 Review.
Cited by
-
Identification of apolipoprotein A-I in BALF as a biomarker of sarcoidosis.Sarcoidosis Vasc Diffuse Lung Dis. 2018;35(1):5-15. doi: 10.36141/svdld.v35i1.5834. Epub 2018 Apr 28. Sarcoidosis Vasc Diffuse Lung Dis. 2018. PMID: 32476874 Free PMC article.
-
TRENDS IN CHOLESTEROL AND LIPOPROTEINS ARE ASSOCIATED WITH ACUTE RESPIRATORY DISTRESS SYNDROME INCIDENCE AND DEATH AMONG SEPSIS PATIENTS.Shock. 2024 Feb 1;61(2):260-265. doi: 10.1097/SHK.0000000000002295. Epub 2023 Dec 28. Shock. 2024. PMID: 38407817 Free PMC article.
-
ATP-binding cassette transporter 1 attenuates ovalbumin-induced neutrophilic airway inflammation.Am J Respir Cell Mol Biol. 2014 Nov;51(5):626-36. doi: 10.1165/rcmb.2013-0264OC. Am J Respir Cell Mol Biol. 2014. PMID: 24813055 Free PMC article.
-
High-density Lipoproteins and Apolipoprotein A-I: Potential New Players in the Prevention and Treatment of Lung Disease.Front Pharmacol. 2016 Sep 21;7:323. doi: 10.3389/fphar.2016.00323. eCollection 2016. Front Pharmacol. 2016. PMID: 27708582 Free PMC article. Review.
-
Effectiveness of Red Watermelon in Preventing Atherosclerosis Through the Role of Lipids, PCSK9, LOX-1, CD36, and ABCA1 in Wistar Rats.Curr Issues Mol Biol. 2025 Jun 8;47(6):433. doi: 10.3390/cimb47060433. Curr Issues Mol Biol. 2025. PMID: 40699832 Free PMC article.
References
-
- McElroy MC, Kasper M. The use of alveolar epithelial type I cell-selective markers to investigate lung injury and repair. Eur Respir J 2004;24:664–673. - PubMed
-
- Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis 1982;126:332–337. - PubMed
-
- Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol 1975;22:142–150. - PubMed
-
- Fuchs S, Hollins AJ, Laue M, Schaefer UF, Roemer K, Gumbleton M, Lehr CM. Differentiation of human alveolar epithelial cells in primary culture: morphological characterization and synthesis of caveolin-1 and surfactant protein-C. Cell Tissue Res 2003;311:31–45. - PubMed
-
- Cheek JM, Evans MJ, Crandall ED. Type I cell-like morphology in tight alveolar epithelial monolayers. Exp Cell Res 1989;184:375–387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

