Chronic paraplegia-induced muscle atrophy downregulates the mTOR/S6K1 signaling pathway
- PMID: 17885021
- PMCID: PMC2715299
- DOI: 10.1152/japplphysiol.00736.2007
Chronic paraplegia-induced muscle atrophy downregulates the mTOR/S6K1 signaling pathway
Abstract
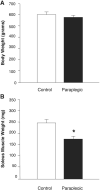
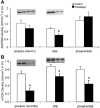
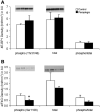
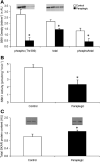
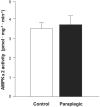
Ribosomal S6 kinase 1 (S6K1) is a downstream component of the mammalian target of rapamycin (mTOR) signaling pathway and plays a regulatory role in translation initiation, protein synthesis, and muscle hypertrophy. AMP-activated protein kinase (AMPK) is a cellular energy sensor, a negative regulator of mTOR, and an inhibitor of protein synthesis. The purpose of this study was to determine whether the hypertrophy/cell growth-associated mTOR pathway was downregulated during muscle atrophy associated with chronic paraplegia. Soleus muscle was collected from male Sprague-Dawley rats 10 wk following complete T(4)-T(5) spinal cord transection (paraplegic) and from sham-operated (control) rats. We utilized immunoprecipitation and Western blotting techniques to measure upstream [AMPK, Akt/protein kinase B (PKB)] and downstream components of the mTOR signaling pathway [mTOR, S6K1, SKAR, 4E-binding protein 1 (4E-BP1), and eukaryotic initiation factor (eIF) 4G and 2alpha]. Paraplegia was associated with significant soleus muscle atrophy (174 +/- 8 vs. 240 +/- 13 mg; P < 0.05). There was a reduction in phosphorylation of mTOR, S6K1, and eIF4G (P < 0.05) with no change in Akt/PKB or 4E-BP1 (P > 0.05). Total protein abundance of mTOR, S6K1, eIF2alpha, and Akt/PKB was decreased, and increased for SKAR (P < 0.05), whereas 4E-BP1 and eIF4G did not change (P > 0.05). S6K1 activity was significantly reduced in the paraplegic group (P < 0.05); however, AMPKalpha2 activity was not altered (3.5 +/- 0.4 vs. 3.7 +/- 0.5 pmol x mg(-1) x min(-1), control vs. paraplegic rats). We conclude that paraplegia-induced muscle atrophy in rats is associated with a general downregulation of the mTOR signaling pathway. Therefore, in addition to upregulation of atrophy signaling during muscle wasting, downregulation of muscle cell growth/hypertrophy-associated signaling appears to be an important component of long-term muscle loss.
Figures
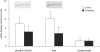
References
-
- Adams GR, Caiozzo VJ, Baldwin KM. Skeletal muscle unweighting: spaceflight and ground-based models. J Appl Physiol. 2003;95:2185–2201. - PubMed
-
- Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol. 1999;276:C120–C127. - PubMed
-
- Baldwin KM, Herrick RE, Ilyina-Kakueva E, Oganov VS. Effects of zero gravity on myofibril content and isomyosin distribution in rodent skeletal muscle. FASEB J. 1990;4:79–83. - PubMed
-
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. - PubMed
-
- Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

