A nonclassical arabinogalactan protein gene highly expressed in vascular tissues, AGP31, is transcriptionally repressed by methyl jasmonic acid in Arabidopsis
- PMID: 17885091
- PMCID: PMC2048811
- DOI: 10.1104/pp.107.102657
A nonclassical arabinogalactan protein gene highly expressed in vascular tissues, AGP31, is transcriptionally repressed by methyl jasmonic acid in Arabidopsis
Abstract
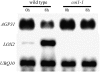
In response to wounding and pathogens, jasmonate (JA) serves as a signal molecule for both induction and repression of gene expression. To examine defense-regulated gene repression in Arabidopsis (Arabidopsis thaliana), we have identified a nonclassical arabinogalactan protein (AGP) gene, AGP31, and show that its mRNA decreased to about 30% of its original level within 8 h in response to methyl JA (MeJA) treatment of whole 7-d-old seedlings. Wounding and abscisic acid treatment had similar effects. MeJA suppression primarily depends on the action of the JA-signaling protein, COI1, as shown by much lower MeJA suppression in coi1-1 mutant plants. The main mechanism of mRNA suppression by MeJA is repression of transcription, as shown by nuclear run-on experiments. The AGP31 protein shares features with several known and putative nonclassical AGPs from other species: a putative signal peptide, a histidine-rich region near the N terminus followed by a repetitive proline-rich domain, and a cysteine-rich C-terminal PAC (for proline-rich protein and AGP, containing cysteine) domain. Positive Yariv reagent interaction demonstrated that the protein is an AGP. Monosaccharide analysis of purified AGP31 indicated it is a galactose-rich AGP. Expression of an AGP31-enhanced green fluorescent protein fusion protein in transgenic cells revealed that the AGP31 protein was localized to the cell wall. AGP31 promoter-beta-glucuronidase reporter gene analysis showed expression in the vascular bundle throughout the plant, except in the flower. In the flower, beta-glucuronidase staining occurred throughout the pistil, except in the stigma. The strong preferential expression in vascular tissues suggests that AGP31 may be involved in vascular tissue function during both the defense response and development.
Figures






References
-
- Ahn JH (2000) RNA extraction for northern blot and RT-PCR. In D Weigel, J Glazebrook, eds, Arabidopsis: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 172–173
-
- Arnim Av (2002) Subcellular localization of GUS- and GFP-tagged protein in onion epidermal cells. In D Weigel, J Glazebrook, eds, Arabidopsis: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 252–257
-
- Baldwin TC, Domingo C, Schindler T, Seetharaman G, Stacey N, Roberts K (2001) DcAGP1, a secreted arabinogalactan protein, is related to a family of basic proline-rich proteins. Plant Mol Biol 45 421–435 - PubMed
-
- Bevilacqua A, Ceriani MC, Capaccioli S, Nicolin A (2003) Post-transcriptional regulation of gene expression by degradation of messenger RNAs. J Cell Physiol 195 356–372 - PubMed
-
- Bomblies K (2000) Whole mount GUS staining. In D Weigel, J Glazebrook, eds, Arabidopsis: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 243–245
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

