Insulin-like growth factor (IGF) binding protein-5 blocks skeletal muscle differentiation by inhibiting IGF actions
- PMID: 17885206
- PMCID: PMC2194633
- DOI: 10.1210/me.2007-0336
Insulin-like growth factor (IGF) binding protein-5 blocks skeletal muscle differentiation by inhibiting IGF actions
Abstract
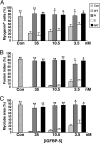
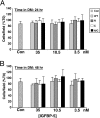
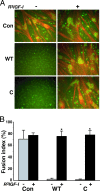
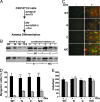
Signaling through the IGF-I receptor by locally produced IGF-I or -II is critical for normal skeletal muscle development and repair after injury. In most tissues, IGF action is modulated by IGF binding proteins (IGFBPs). IGFBP-5 is produced by muscle cells, and previous studies have suggested that when overexpressed it may either facilitate or inhibit IGF actions, and thus potentially enhance or diminish IGF-mediated myoblast differentiation or survival. To resolve these contradictory observations and discern the mechanisms of action of IGFBP-5, we studied its effects in cultured muscle cells. Purified wild-type (WT) mouse IGFBP-5 or a variant with diminished extracellular matrix binding (C domain mutant) each prevented differentiation at final concentrations as low as 3.5 nm, whereas analogs with reduced IGF binding (N domain mutant) were ineffective even at 100 nm. None of the IGFBP-5 variants altered cell number. An IGF-I analog (R(3)IGF-I) with diminished affinity for IGFBPs promoted full muscle differentiation in the presence of IGFBP-5(WT), showing that IGFBP-5 interferes with IGF-dependent signaling pathways in myoblasts. When IGFBP-5(WT) or variants were overexpressed by adenovirus-mediated gene transfer, concentrations in muscle culture medium reached 500 nm, and differentiation was inhibited, even by IGFBP-5(N). As 200 nm of purified IGFBP-5(N) prevented activation of the IGF-I receptor by 10 nm IGF-II as effectively as 2 nm of IGFBP-5(WT), our results not only demonstrate that IGFBP-5 variants with reduced IGF binding affinity impair muscle differentiation by blocking IGF actions, but underscore the need for caution when labeling effects of IGFBPs as IGF independent because even low-affinity analogs may potently inhibit IGF-I or -II if present at high enough concentrations in biological fluids.
Figures




Similar articles
-
Modulation of insulin-like growth factor actions in L6A1 myoblasts by insulin-like growth factor binding protein (IGFBP)-4 and IGFBP-5: a dual role for IGFBP-5.J Cell Physiol. 1998 Oct;177(1):47-57. doi: 10.1002/(SICI)1097-4652(199810)177:1<47::AID-JCP5>3.0.CO;2-E. J Cell Physiol. 1998. PMID: 9731744
-
Insulin-like growth factor-binding protein-5 inhibits osteoblast differentiation and skeletal growth by blocking insulin-like growth factor actions.Mol Endocrinol. 2008 May;22(5):1238-50. doi: 10.1210/me.2008-0001. Epub 2008 Feb 21. Mol Endocrinol. 2008. PMID: 18292241 Free PMC article.
-
Skeletal muscle cell-derived insulin-like growth factor (IGF) binding proteins inhibit IGF-I-induced myogenesis in rat L6E9 cells.Endocrinology. 1995 Feb;136(2):720-6. doi: 10.1210/endo.136.2.7530651. Endocrinology. 1995. PMID: 7530651
-
Growth hormone and the insulin-like growth factor system in myogenesis.Endocr Rev. 1996 Oct;17(5):481-517. doi: 10.1210/edrv-17-5-481. Endocr Rev. 1996. PMID: 8897022 Review.
-
Insulin-like growth factor binding proteins initiate cell death and extracellular matrix remodeling in the mammary gland.Domest Anim Endocrinol. 2005 Aug;29(2):274-82. doi: 10.1016/j.domaniend.2005.02.021. Epub 2005 Apr 7. Domest Anim Endocrinol. 2005. PMID: 15998501 Review.
Cited by
-
Defining the disulfide bonds of insulin-like growth factor-binding protein-5 by tandem mass spectrometry with electron transfer dissociation and collision-induced dissociation.J Biol Chem. 2012 Jan 6;287(2):1510-9. doi: 10.1074/jbc.M111.285528. Epub 2011 Nov 22. J Biol Chem. 2012. PMID: 22117064 Free PMC article.
-
Identifying growth hormone-regulated enhancers in the Igf1 locus.Physiol Genomics. 2015 Nov;47(11):559-68. doi: 10.1152/physiolgenomics.00062.2015. Epub 2015 Sep 1. Physiol Genomics. 2015. PMID: 26330488 Free PMC article.
-
Investigating endogenous peptides and peptidases using peptidomics.Biochemistry. 2011 Sep 6;50(35):7447-61. doi: 10.1021/bi200417k. Epub 2011 Aug 15. Biochemistry. 2011. PMID: 21786763 Free PMC article. Review.
-
Habitual exercise plus dietary supplementation with milk fat globule membrane improves muscle function deficits via neuromuscular development in senescence-accelerated mice.Springerplus. 2014 Jul 4;3:339. doi: 10.1186/2193-1801-3-339. eCollection 2014. Springerplus. 2014. PMID: 25110626 Free PMC article.
-
Comprehensive analysis of lncRNAs and mRNAs in skeletal muscle of rainbow trout (Oncorhynchus mykiss) exposed to estradiol.Sci Rep. 2017 Sep 18;7(1):11780. doi: 10.1038/s41598-017-12136-6. Sci Rep. 2017. PMID: 28924252 Free PMC article.
References
-
- Buckingham M 2001 Skeletal muscle formation in vertebrates. Curr Opin Genet Dev 11:440–448 - PubMed
-
- Berkes CA, Tapscott SJ 2005 MyoD and the transcriptional control of myogenesis. Semin Cell Dev Biol 16:585–595 - PubMed
-
- Charge SB, Rudnicki MA 2004 Cellular and molecular regulation of muscle regeneration. Physiol Rev 84:209–238 - PubMed
-
- Glass DJ 2003 Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol 5:87–90 - PubMed
-
- Rotwein P 2003 Insulin-like growth factor action and skeletal muscle growth, an in vivo perspective. Growth Horm IGF Res 13:303–305 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

