Angiogenin loss-of-function mutations in amyotrophic lateral sclerosis
- PMID: 17886298
- PMCID: PMC2776820
- DOI: 10.1002/ana.21221
Angiogenin loss-of-function mutations in amyotrophic lateral sclerosis
Abstract
Objective: Heterozygous missense mutations in the coding region of angiogenin (ANG), an angiogenic ribonuclease, have been reported in amyotrophic lateral sclerosis (ALS) patients. However, the role of ANG in motor neuron physiology and the functional consequences of these mutations are unknown. We searched for new mutations and sought to define the functional consequences of these mutations.
Methods: We sequenced the coding region of ANG in an independent cohort of North American ALS patients. Identified ANG mutations were then characterized using functional assays of angiogenesis, ribonucleolysis, and nuclear translocation. We also examined expression of ANG in normal human fetal and adult spinal cords.
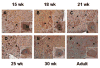
Results: We identified four mutations in the coding region of ANG from 298 ALS patients. Three of these mutations are present in the mature protein. Among the four mutations, P(-4)S, S28N, and P112L are novel, and K17I has been reported previously. Functional assays show that these ANG mutations result in complete loss of function. The mutant ANG proteins are unable to induce angiogenesis because of a deficiency in ribonuclease activity, nuclear translocation, or both. As a correlate, we demonstrate strong ANG expression in both endothelial cells and motor neurons of normal human spinal cords from the developing fetus and adult.
Interpretation: We provide the first evidence that ANG mutations, identified in ALS patients, are associated with functional loss of ANG activity. Moreover, strong ANG expression, in normal human fetal and adult spinal cord neurons and endothelial cells, confirms the plausibility of ANG dysfunction being relevant to the pathogenesis of ALS.
Figures




Similar articles
-
Identification of novel Angiogenin (ANG) gene missense variants in German patients with amyotrophic lateral sclerosis.J Neurol. 2009 Aug;256(8):1337-42. doi: 10.1007/s00415-009-5124-4. Epub 2009 Apr 12. J Neurol. 2009. PMID: 19363631 Free PMC article.
-
Mechanisms of loss of functions of human angiogenin variants implicated in amyotrophic lateral sclerosis.PLoS One. 2012;7(2):e32479. doi: 10.1371/journal.pone.0032479. Epub 2012 Feb 27. PLoS One. 2012. PMID: 22384259 Free PMC article.
-
Targeting angiogenin in therapy of amyotropic lateral sclerosis.Expert Opin Ther Targets. 2008 Oct;12(10):1229-42. doi: 10.1517/14728222.12.10.1229. Expert Opin Ther Targets. 2008. PMID: 18781822 Free PMC article. Review.
-
Lack of unique neuropathology in amyotrophic lateral sclerosis associated with p.K54E angiogenin (ANG) mutation.Neuropathol Appl Neurobiol. 2013 Aug;39(5):562-71. doi: 10.1111/nan.12007. Neuropathol Appl Neurobiol. 2013. PMID: 23228179 Free PMC article.
-
Effects of Pathogenic Mutants of the Neuroprotective RNase 5-Angiogenin in Amyotrophic Lateral Sclerosis (ALS).Genes (Basel). 2024 Jun 4;15(6):738. doi: 10.3390/genes15060738. Genes (Basel). 2024. PMID: 38927674 Free PMC article. Review.
Cited by
-
Ribonuclease/angiogenin inhibitor 1 regulates stress-induced subcellular localization of angiogenin to control growth and survival.J Cell Sci. 2013 Sep 15;126(Pt 18):4308-19. doi: 10.1242/jcs.134551. Epub 2013 Jul 10. J Cell Sci. 2013. PMID: 23843625 Free PMC article.
-
Quantification of the Relative Contributions of Loss-of-function and Gain-of-function Mechanisms in TAR DNA-binding Protein 43 (TDP-43) Proteinopathies.J Biol Chem. 2016 Sep 9;291(37):19437-48. doi: 10.1074/jbc.M116.737726. Epub 2016 Jul 21. J Biol Chem. 2016. PMID: 27445339 Free PMC article.
-
Transcription of angiogenin and ribonuclease 4 is regulated by RNA polymerase III elements and a CCCTC binding factor (CTCF)-dependent intragenic chromatin loop.J Biol Chem. 2014 May 2;289(18):12520-34. doi: 10.1074/jbc.M114.551762. Epub 2014 Mar 21. J Biol Chem. 2014. PMID: 24659782 Free PMC article.
-
Identification of novel Angiogenin (ANG) gene missense variants in German patients with amyotrophic lateral sclerosis.J Neurol. 2009 Aug;256(8):1337-42. doi: 10.1007/s00415-009-5124-4. Epub 2009 Apr 12. J Neurol. 2009. PMID: 19363631 Free PMC article.
-
Control of motoneuron survival by angiogenin.J Neurosci. 2008 Dec 24;28(52):14056-61. doi: 10.1523/JNEUROSCI.3399-08.2008. J Neurosci. 2008. PMID: 19109488 Free PMC article.
References
-
- Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–1700. - PubMed
-
- Gros-Louis F, Gaspar C, Rouleau GA. Genetics of familial and sporadic amyotrophic lateral sclerosis. Biochim Biophys Acta. 2006;1762:956–972. - PubMed
-
- Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006;7:710–723. - PubMed
-
- Boillee S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. - PubMed
-
- Andersen PM, Nilsson P, Keranen ML, et al. Phenotypic heterogeneity in motor neuron disease patients with CuZn-superoxide dismutase mutations in Scandinavia. Brain. 1997;120(pt 10):1723–1737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

